163619
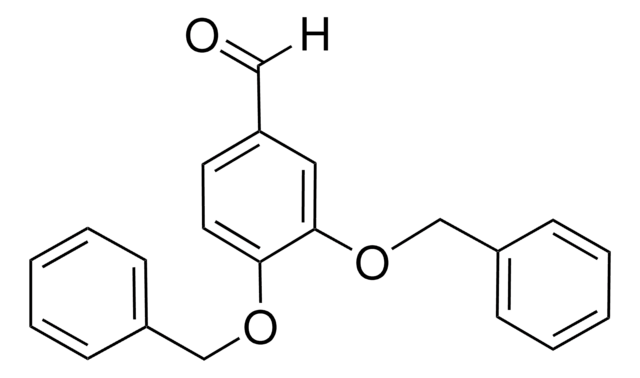
4-Benzyloxy-3-methoxybenzaldehyde
98%
Synonym(s):
O-Benzylvanillin, Vanillin benzyl ether
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2OC6H3(OCH3)CHO
CAS Number:
Molecular Weight:
242.27
Beilstein:
1464258
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
62-64 °C (lit.)
functional group
aldehyde
phenyl
SMILES string
[H]C(=O)c1ccc(OCc2ccccc2)c(OC)c1
InChI
1S/C15H14O3/c1-17-15-9-13(10-16)7-8-14(15)18-11-12-5-3-2-4-6-12/h2-10H,11H2,1H3
InChI key
JSHLOPGSDZTEGQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Benzyloxy-3-methoxybenzaldehyde reacts with benzohydrazide to yield (E)-N′-(4-benzyloxy-3-methoxybenzylidene)benzohydrazide.
Application
4-Benzyloxy-3-methoxybenzaldehyde was used in the synthesis of 1,2-bis(4-benzyloxy-3-methoxyphenyl)-3-hydroxy-propionic acid. It was also used in first enantioselective total synthesis of a neurotrophic (-)-talaumidin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
(E)-N'-(4-Benzyloxy-3-methoxybenzylidene) benzohydrazide.
He Y-Z and Liu D-Z.
Acta Crystallographica Section E, Structure Reports Online, 61(11), o3855-o3856 (2005)
First enantioselective synthesis of (-)-talaumidin, a neurotrophic diaryltetrahydrofuran-type lignan.
Esumi T, et al.
Tetrahedron Letters, 47(24), 3979-3983 (2006)
Synthesis of the erythro and threo forms of 1, 2-bis (4-hydroxy-3-methoxyphenyl)-l, 3-propanediol.
Berndtsson L, et al.
Acta Chemica Scandinavica. Series B, 34, 453-455 (1980)
Joanna Kozłowska et al.
Molecules (Basel, Switzerland), 24(22) (2019-11-17)
A series of 18 aminochalcone derivatives were obtained in yields of 21.5-88.6% by applying the classical Claisen-Schmidt reaction. Compounds 4-9, 14 and 16-18 with 4-ethyl, 4-carboxy-, 4-benzyloxy- and 4-benzyloxy-3-methoxy groups were novel, not previously described in the scientific literature. To
C A Jackson et al.
Journal of applied microbiology, 122(4), 940-952 (2017-01-17)
The aim of this work was to isolate novel lignin-degrading organisms. Several pure cultures of bacteria that degrade lignin were isolated from bacterial consortia developed from decaying biomass. Among the isolates, Rhizobium sp. strain YS-1r (closest relative of Rhizobium petrolearium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service