129488
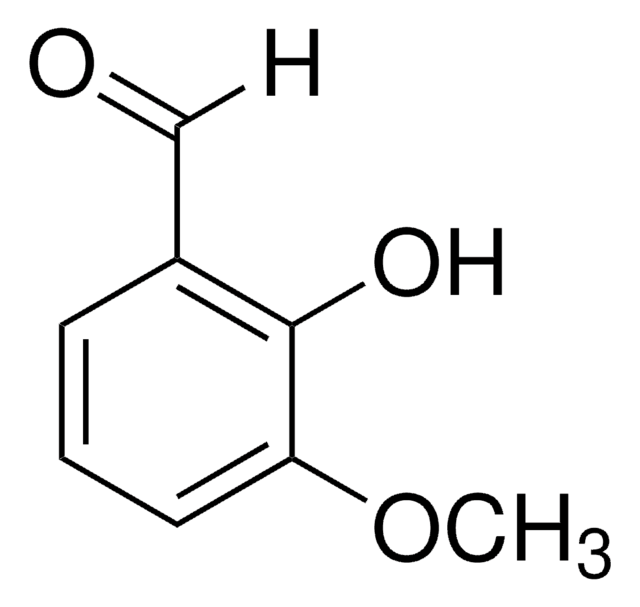
5-Iodovanillin
97%
Synonym(s):
4-Hydroxy-3-iodo-5-methoxybenzaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
IC6H2(OCH3)(OH)CHO
CAS Number:
Molecular Weight:
278.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
183-185 °C (lit.)
functional group
aldehyde
iodo
SMILES string
COc1cc(C=O)cc(I)c1O
InChI
1S/C8H7IO3/c1-12-7-3-5(4-10)2-6(9)8(7)11/h2-4,11H,1H3
InChI key
FBBCSYADXYILEH-UHFFFAOYSA-N
Application
5-Lodovanillin was used in the characterization of 5-Iodoacetovanillone. 5-Iodovanillin has also been used in the synthesis of platelet activating factor antagonist L-659,989.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thompson AS, et al.
Tetrahedron Letters, 31(48), 6953-6956 (1990)
An improved synthesis of acetosyringone.
Crawford LW, et al.
Canadian Journal of Chemistry, 34(11), 1562-1566 (1956)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service