V1377
Vinblastin -sulfat (Salz)
≥97% (HPLC), powder, plant alkaloid
Synonym(e):
VLB, Vincaleukoblastin -sulfat (Salz)
About This Item
Empfohlene Produkte
product name
Vinblastin -sulfat (Salz), ≥97% (HPLC)
Qualitätsniveau
Assay
≥97% (HPLC)
Form
(powder or amorphous or crystalline powder)
Farbe
white to light yellow
mp (Schmelzpunkt)
267 °C (dec.) (lit.)
Absorption
14 at 270 nm in 0.1 M phosphate buffer at 1 mM
16.2 at 259 nm in ethanol at 1 mM
53.7 at 214 nm in ethanol at 1 mM
Wirkungsspektrum von Antibiotika
neoplastics
Wirkungsweise
DNA synthesis | interferes
Ersteller
Eli Lilly
Lagertemp.
2-8°C
SMILES String
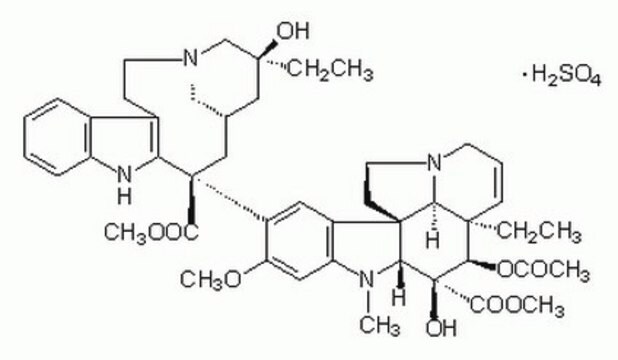
OS(O)(=O)=O.[H][C@@]12CN(CCc3c([nH]c4ccccc34)[C@@](C1)(C(=O)OC)c5cc6c(cc5OC)N(C)[C@@]7([H])[C@](O)([C@H](OC(C)=O)[C@]8(CC)C=CCN9CC[C@]67[C@]89[H])C(=O)OC)C[C@](O)(CC)C2
InChI
1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37-,38+,39+,42-,43+,44+,45-,46-;/m0./s1
InChIKey
KDQAABAKXDWYSZ-PNYVAJAMSA-N
Angaben zum Gen
human ... TBCC(6903) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- as a microtubule depolymerizing drug for the synchronization of human cell lines in G2/M phase
- as a multidrug resistance screening substrate in human colon cancer cell line (HCT116) cell line
- as an antimicrotubule agent in sub perineural glia of Drosophila brain
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Muta. 2 - Repr. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
We presents an article on ABC Transporters and Cancer Drug Resistance
Verwandter Inhalt
Discover Bioactive Small Molecules for ADME/Tox
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.