Wichtige Dokumente
L9283
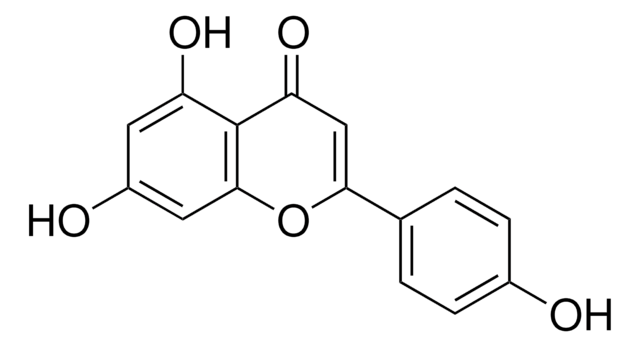
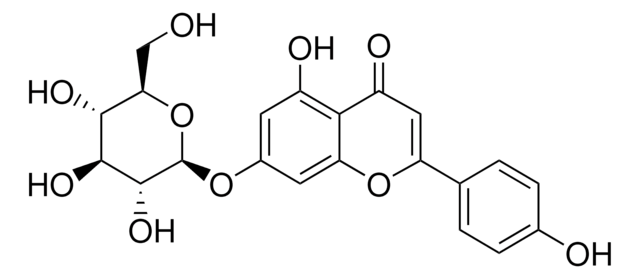
Luteolin
≥98% (TLC), powder, antioxidant
Synonym(e):
3′,4′,5,7-Tetrahydroxyflavon
About This Item
Empfohlene Produkte
Produktbezeichnung
Luteolin, ≥98% (TLC), powder
Qualitätsniveau
Assay
≥98% (TLC)
Form
powder
Haltbarkeit
3 yr
Farbe
yellow
mp (Schmelzpunkt)
~330 °C (lit.)
Lagertemp.
2-8°C
SMILES String
Oc1cc(O)c2C(=O)C=C(Oc2c1)c3ccc(O)c(O)c3
InChI
1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H
InChIKey
IQPNAANSBPBGFQ-UHFFFAOYSA-N
Angaben zum Gen
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , GSK3A(2931)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- to induce and elucidate the apoptotic pathway in renal cell carcinoma 786-O cells
- as an additive in M9 minimal medium to induce nodF gene expression
- as a reference standard to qualitatively and quantitatively analyse luteolin using reverse phase-high performance liquid chromatography with diode array detector (RP-HPLC-DAD)
- as a reaction supplement for β-galactosidase assay
- to elucidate the anti-inflammatory efficacy of luteolin in pseudorabies virus infected RAW264.7 cell line by measuring the anti-inflammatory mediators production and also cell viability and cytotoxicity assay
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Verwandter Inhalt
DISCOVER Bioactive Small Molecules for Nitric Oxide & Cell Stress Research
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.