L6250
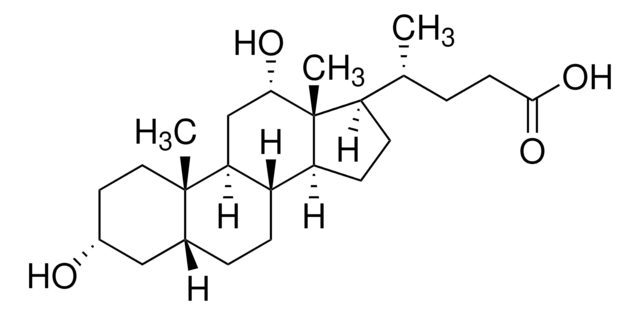
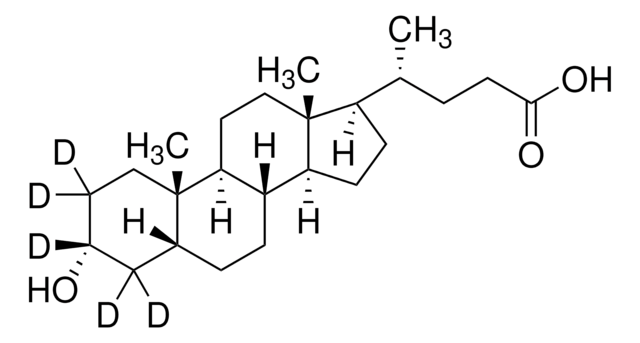
Lithocholsäure
≥95%
Synonym(e):
3α-Hydroxy-5β-cholan-24-säure, 3α-Hydroxy-5β-cholansäure, 5β-Cholan-24-säure-3α-ol
About This Item
Empfohlene Produkte
Biologische Quelle
bovine bile
synthetic
Qualitätsniveau
Assay
≥95%
Mol-Gew.
376.57 g/mol
mp (Schmelzpunkt)
183-188 °C (lit.)
Funktionelle Gruppe
carboxylic acid
Versandbedingung
ambient
Lagertemp.
room temp
SMILES String
[H][C@]12CC[C@@]3([H])[C@]4([H])CC[C@H]([C@H](C)CCC(O)=O)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@@H](O)C2
InChI
1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1
InChIKey
SMEROWZSTRWXGI-HVATVPOCSA-N
Angaben zum Gen
human ... POLA1(5422) , TOP2A(7153)
rat ... Polb(29240)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
The liver excretes excess cholesterol in the form of bile acids. Bile acids serve two purposes: to remove unwanted cholesterol from the body and to aid in lipid digestion in the intestine.
Protokolle
This method is particularly useful in research into the role of individual bile acids as signaling molecules; suitable for clinical laboratories to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
Verwandter Inhalt
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.