Alle Fotos(2)
Wichtige Dokumente
D9150
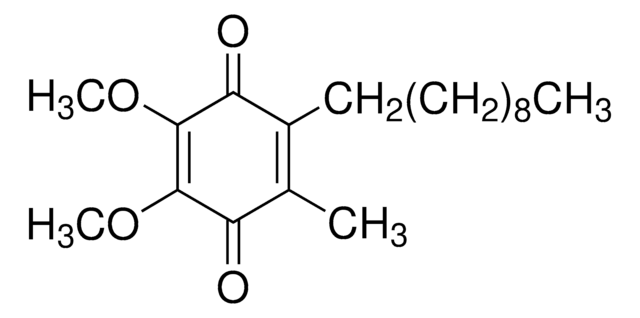
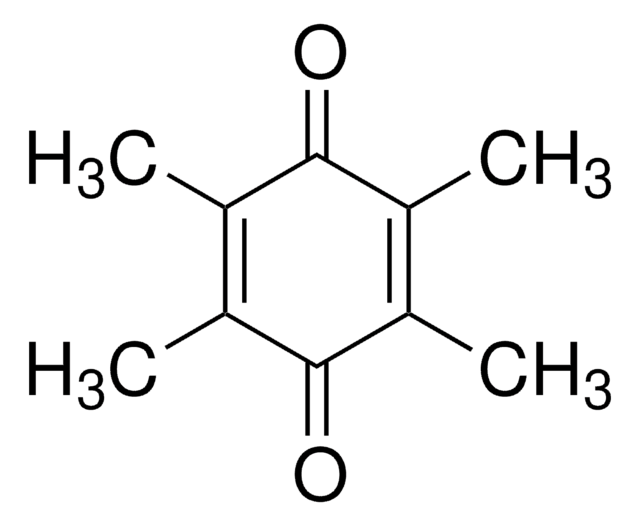
2,3-Dimethoxy-5-Methyl-p-Benzochinon
apoptosis inducer
Synonym(e):
Coenzym Q0
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C9H10O4
CAS-Nummer:
Molekulargewicht:
182.17
Beilstein:
1640422
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352204
PubChem Substanz-ID:
NACRES:
NA.51
Empfohlene Produkte
Qualitätsniveau
Form
powder
mp (Schmelzpunkt)
58-60 °C (lit.)
Lagertemp.
2-8°C
SMILES String
COC1=C(OC)C(=O)C(C)=CC1=O
InChI
1S/C9H10O4/c1-5-4-6(10)8(12-2)9(13-3)7(5)11/h4H,1-3H3
InChIKey
UIXPTCZPFCVOQF-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
2,3-Dimethoxy-5-methyl-p-benzoquinone (Coenzyme Q0 or DMM) is present in all the cells including neural cells.
Anwendung
2,3-Dimethoxy-5-methyl-p-benzoquinone has been used:
- as a tau protein fibrillization inducer to determine the regions of tau involved in the formation of paired helical filaments (PHFs)
- as a component in buffer B for cytochrome oxidation assay with subsaturating light
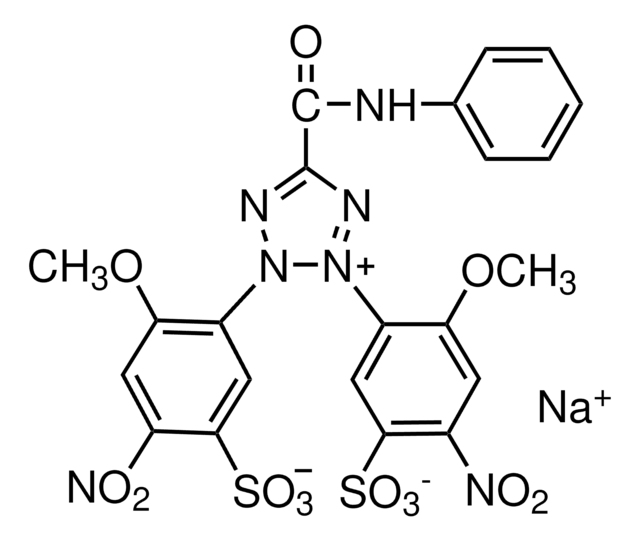
- in the RPMI-1640 medium for 2,3-bis-(2-methoxy-4-nitro-5-sulphenyl)-(2H)-tetrazolium-5-carboxanilide (XTT) assay to quantify antifungal activity
Coenzyme Q0 inhibits (via radical quenching) reactions of gamma-irradiation induced homolytic cleavage of O-glycoside bonds in polysaccharides. Coenzyme Q0 induces apoptosis and modulates the cell cycle in estrogen receptor negative breast cancer cells. It is toxic to other cells such as insulin producing cells.
Biochem./physiol. Wirkung
2,3-Dimethoxy-5-methyl-p-benzoquinone (Coenzyme Q0) interacts with tau protein and aids in the formation of filamentous structure.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
XTT assay of antifungal activity
Loures FV and Levitz SM
PLoS Pathogens, 5(15), e1543-e1543 (2015)
The Photosynthetic Bacterial Reaction Center: Structure and Dynamics, 114-114 (2013)
Augustin C Mot et al.
PloS one, 15(1), e0225530-e0225530 (2020-01-22)
Yellow laccases lack the typical blue type 1 Cu absorption band around 600 nm; however, multi-copper oxidases with laccase properties have been reported. We provide the first evidence that the yellow laccase isolated from Sclerotinia sclerotiorum is obtained from a
In vitro tau fibrillization: mapping protein regions
Santa-Maria I, et al.
Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1762(7), 683-692 (2006)
Yung-Fu Wang et al.
Bioelectrochemistry (Amsterdam, Netherlands), 69(1), 74-81 (2006-01-25)
Bioelectrocatalytic oxidation of acetate was investigated under anaerobic conditions by using Escherichia coli K-12 (IFO 3301) cells cultured on aerobic media containing poly-peptone, glucose or acetate as the sole carbon source. It was found that all E. coli cells cultured
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.