Wichtige Dokumente
C7495
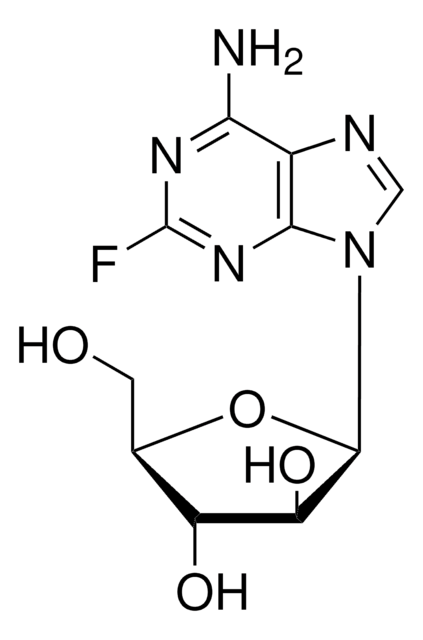
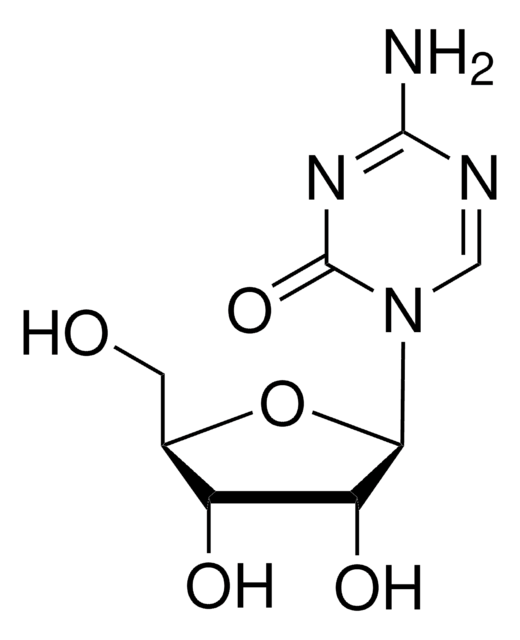
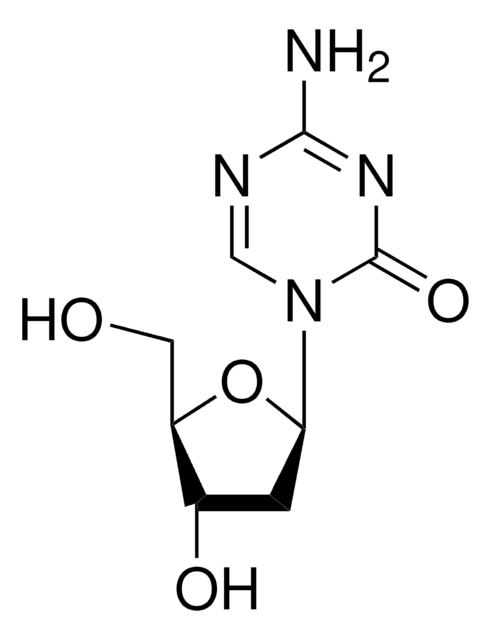
Clofarabine
≥98% (HPLC)
Synonym(e):
(2R,3R,4S,5R)-5-(6-amino-2-chloropurin-9-yl)-4-fluoro-2-(hydroxymethyl)oxolan-3-ol, 2-chloro-2′-arabino-fluoro-2′-deoxyadenosine
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Farbe
white
Löslichkeit
DMSO: >10 mg/mL
Ersteller
Sanofi Aventis
Lagertemp.
2-8°C
SMILES String
Nc1nc(Cl)nc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3F
InChI
1S/C10H11ClFN5O3/c11-10-15-7(13)5-8(16-10)17(2-14-5)9-4(12)6(19)3(1-18)20-9/h2-4,6,9,18-19H,1H2,(H2,13,15,16)/t3-,4+,6-,9-/m1/s1
InChIKey
WDDPHFBMKLOVOX-AYQXTPAHSA-N
Angaben zum Gen
human ... POLA1(5422) , POLA2(23649) , POLD1(5424) , POLD2(5425) , POLD3(10714) , POLD4(57804) , POLE(5426) , POLE2(5427) , POLE3(54107) , PRIM1(5557) , PRIM2(5558) , RRM1(6240) , RRM2(6241) , RRM2B(50484)
Anwendung
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Signalwort
Warning
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Verwandter Inhalt
Apoptosis, or programmed cell death (PCD), is a selective process for the removal of unnecessary, infected or transformed cells in various biological systems. As it plays a role in the homeostasis of multicellular organisms, apoptosis is tightly regulated through two principal pathways by a number of regulatory and effector molecules.
n proliferating cells, the cell cycle consists of four phases. Gap 1 (G1) is the interval between mitosis and DNA replication that is characterized by cell growth. Replication of DNA occurs during the synthesis (S) phase, which is followed by a second gap phase (G2) during which growth and preparation for cell division occurs. Together, these three stages comprise the interphase phase of the cell cycle. Interphase is followed by the mitotic (M) phase.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.