C0737
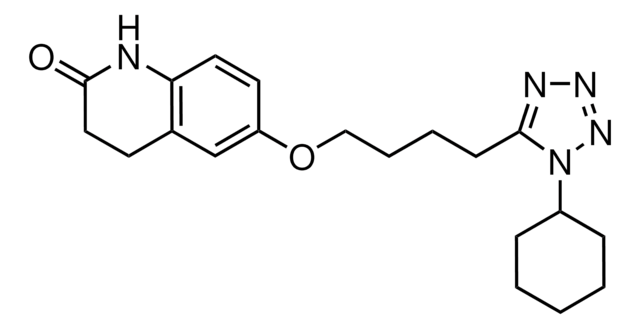
Cilostazol
≥98% (HPLC), powder
Synonym(e):
6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)-butoxy]-3,4-dihydro-2(1H)-chinolinon, OPC 13013, OPC 21, Pletaal
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥98% (HPLC)
Form
powder
Farbe
off-white
Löslichkeit
DMSO: 10 mg/mL, clear
Ersteller
Otsuka Pharma
SMILES String
O=C1CCc2cc(OCCCCc3nnnn3C4CCCCC4)ccc2N1
InChI
1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
InChIKey
RRGUKTPIGVIEKM-UHFFFAOYSA-N
Angaben zum Gen
human ... PDE3A(5139) , PDE3B(5140)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- to reduce Madin–Darby cell line (MDCK) proliferation through c-Myc down-regulation
- in the in vitro assessment of toxin delivery in T84 intestinal epithelial cells
- to induce adenosine triphosphate (ATP) release in white adipocytes
Biochem./physiol. Wirkung
Leistungsmerkmale und Vorteile
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Repr. 2
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Verwandter Inhalt
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.