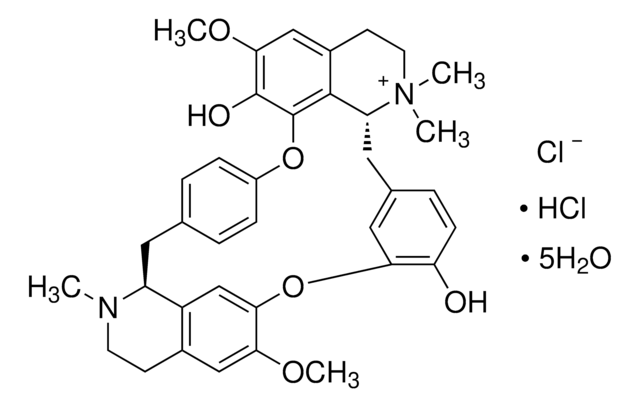
93750
(+)-Tubocurarinchlorid Pentahydrat
≥97.0% (TLC)
Synonym(e):
(+)-Tubocurarine chloride hydrochloride pentahydrate, D-Tubocurarine dichloride pentahydrate, Tubarine pentahydrate
About This Item
Empfohlene Produkte
Biologische Quelle
plant
Assay
≥97.0% (TLC)
Form
powder
Optische Aktivität
[α]20/D +195±5°, c = 0.5% in H2O
Verunreinigungen
~12% water
mp (Schmelzpunkt)
275-280 °C (dec.) (lit.)
Lagertemp.
2-8°C
SMILES String
[Cl-].Cl[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].[H]O[H].COc1cc2CCN(C)[C@H]3Cc4ccc(Oc5c(O)c(OC)cc6CC[N+](C)(C)[C@H](Cc7ccc(O)c(Oc1cc23)c7)c56)cc4
InChI
1S/C37H40N2O6.2ClH.5H2O/c1-38-14-12-24-19-32(42-4)33-21-27(24)28(38)16-22-6-9-26(10-7-22)44-37-35-25(20-34(43-5)36(37)41)13-15-39(2,3)29(35)17-23-8-11-30(40)31(18-23)45-33;;;;;;;/h6-11,18-21,28-29H,12-17H2,1-5H3,(H-,40,41);2*1H;5*1H2/t28-,29+;;;;;;;/m0......./s1
InChIKey
WMIZITXEJNQAQK-GGDSLZADSA-N
Angaben zum Gen
human ... CHRNA1(1134) , CHRNB1(1140) , CHRND(1144) , CHRNE(1145) , CHRNG(1146)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- as an acetylcholine receptor antagonist in synapse blocking experiment to study its effect on neuromuscular junction formation (NMJ) formation in a co-culture system of human skeletal muscles and human stem cell-derived motoneurons
- to induce paralysis in zebrafish larvae to study whole-brain imaging of seizures by two-photon light-sheet microscopy
- to block NMJ to study its role in myotube contraction
Biochem./physiol. Wirkung
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 3 Oral
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.