PHR1464
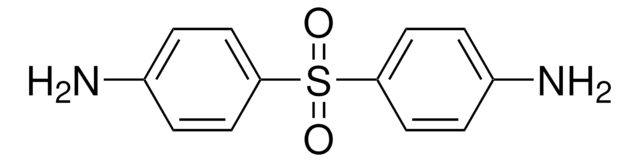
Dapson
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(e):
4-Aminophenylsulfon, 4,4′-Diaminodiphenylsulfon, 4,4′-Sulfonyldianilin, Bis-(4-aminophenyl)-sulfon, DDS, Dapson
About This Item
Empfohlene Produkte
Qualität
certified reference material
pharmaceutical secondary standard
Qualitätsniveau
Agentur
traceable to BP 102
traceable to Ph. Eur. D0100000
traceable to USP 1164008
API-Familie
dapsone
Analysenzertifikat (CofA)
current certificate can be downloaded
Verpackung
pkg of 1 g
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
mp (Schmelzpunkt)
175-177 °C (lit.)
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-30°C
SMILES String
Nc1ccc(cc1)S(=O)(=O)c2ccc(N)cc2
InChI
1S/C12H12N2O2S/c13-9-1-5-11(6-2-9)17(15,16)12-7-3-10(14)4-8-12/h1-8H,13-14H2
InChIKey
MQJKPEGWNLWLTK-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Anwendung
Hinweis zur Analyse
Sonstige Hinweise
Fußnote
Empfohlene Produkte
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Chronic 2 - Repr. 1B - STOT RE 2 - STOT SE 2
Zielorgane
Blood, Blood,spleen,Liver
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.