5.31372
Caspase-1/4 Inhibitor, VX-765
Synonym(e):
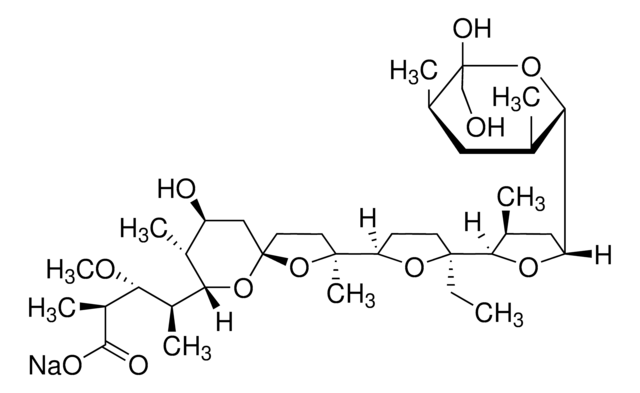
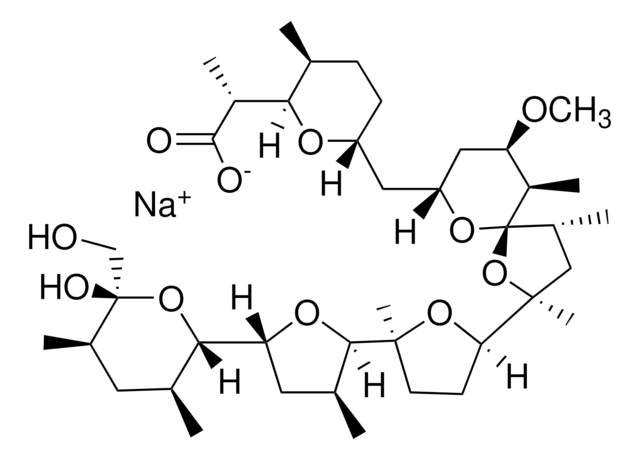
Caspase-1/4 Inhibitor, VX-765, ( S)-1-(( S)-2-((1-(4-Amino-3-chloro-phenyl)-methanoyl)-amino)-3,3-dimethyl-butanoyl)-pyrrolidine-2-carboxylic acid ((2 R,3 S)-2-ethoxy-5-oxo-tetrahydro-furan-3-yl)-amide, ( S)-1-(( S)-2-(4-Amino-3-chlorobenzamid
About This Item
Empfohlene Produkte
Assay
≥98% (HPLC)
Qualitätsniveau
Form
solid
Hersteller/Markenname
Calbiochem®
Lagerbedingungen
OK to freeze
desiccated
protect from light
Farbe
white
Löslichkeit
DMSO: 100 mg/mL
Lagertemp.
−20°C
InChI
1S/C24H33ClN4O6/c1-5-34-23-16(12-18(30)35-23)27-21(32)17-7-6-10-29(17)22(33)19(24(2,3)4)28-20(31)13-8-9-15(26)14(25)11-13/h8-9,11,16-17,19,23H,5-7,10,12,26H2,1-4H3,(H,27,32)(H,28,31)/t16-,17-,19+,23+/m0/s1
InChIKey
SJDDOCKBXFJEJB-MOKWFATOSA-N
Allgemeine Beschreibung
Please note that the molecular weight for this compound is batch-specific due to variable water content.
Biochem./physiol. Wirkung
caspase-1/4
Verpackung
Warnhinweis
Rekonstituierung
Sonstige Hinweise
Wannamaker, W., et al. 2007. J. Pharmacol. Exp. Ther.321, 509.
Ravizza, T., et al. 2006. Epilepsia47, 1160.
Stack, J.H., et al. 2005. J. Immunol.175, 2630.
Rechtliche Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.