N17602
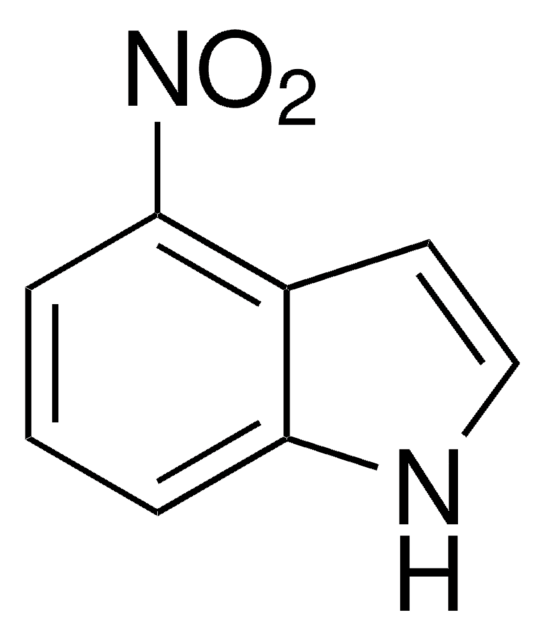
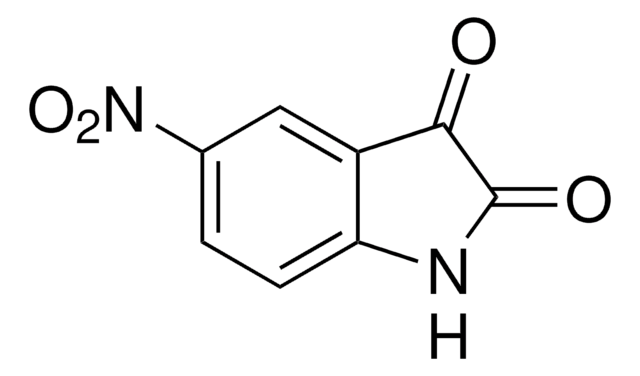
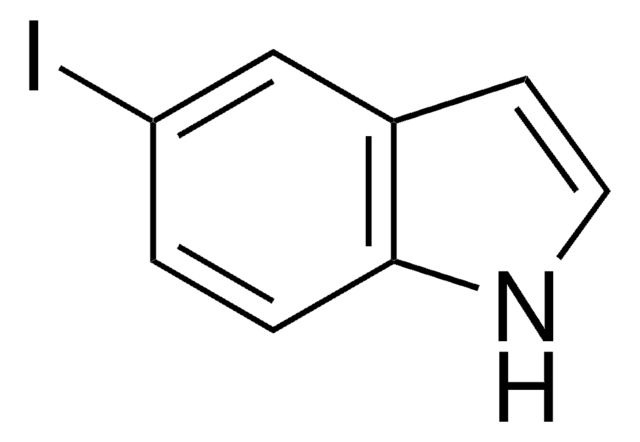
5-Nitroindol
98%
Synonym(e):
NSC 520594
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C8H6N2O2
CAS-Nummer:
Molekulargewicht:
162.15
Beilstein:
383777
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
powder
mp (Schmelzpunkt)
140-142 °C (lit.)
SMILES String
[O-][N+](=O)c1ccc2[nH]ccc2c1
InChI
1S/C8H6N2O2/c11-10(12)7-1-2-8-6(5-7)3-4-9-8/h1-5,9H
InChIKey
OZFPSOBLQZPIAV-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Protein Kinase Inhibitors and antiproliferative agents
- Positive Allosteric Modulators of Metabotropic Glutamate Receptor 4 (mGlu4)
- Antifungal agents
- Cannabinoid receptor type 1 (CB1) antagonists
- Potential anticancer agents
- Potential antivascular agents
- Selective Anti-leukemic agents
- Anti human immunodeficiency virus subtype 1 (HIV-1) agents
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Dam. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
José Gallego et al.
Nucleic acids research, 35(9), 2904-2912 (2007-04-18)
Universal bases hybridize with all other natural DNA or RNA bases, and have applications in PCR and sequencing. We have analysed by nuclear magnetic resonance spectroscopy the structure and dynamics of three DNA oligonucleotides containing the universal base analogues 5-nitroindole
H Challa et al.
Organic letters, 1(10), 1639-1641 (2000-06-03)
[formula: see text] The syntheses of PNA oligomers containing potential ambiguous nucleobase analogues, namely 3-nitropyrrole and 5-nitroindole, have been accomplished. Hybridization properties of these PNAs with complementary oligodeoxynucleotides were evaluated by thermal denaturation experiments. Both novel residues exhibited little variation
P M Vallone et al.
Nucleic acids research, 27(17), 3589-3596 (1999-08-14)
Effects of the universal base 5-nitroindole on the thermodynamic stability of DNA hairpins having a 6 bp stem and four base loops were investigated by optical absorbance and differential scanning calorimetry techniques. Melting studies were conducted in buffer containing 115
Use of 5-nitroindole-2'-deoxyribose-5'-triphosphate for labelling and detection of oligonucleotides.
C L Smith et al.
Nucleosides & nucleotides, 17(1-3), 555-564 (1998-08-26)
The 5'-triphosphate of 5-nitroindole-2'-deoxyriboside has been shown to be a good substrate for terminal deoxynucleotidyl transferase (TdT). An antibody has been prepared for the detection of 5-nitroindole and has been used for the detection of 5-nitroindole tailed DNA both in
David Loakes et al.
Journal of the American Chemical Society, 131(41), 14827-14837 (2009-09-26)
Hydrophobic base analogues (HBAs) have shown great promise for the expansion of the chemical and coding potential of nucleic acids but are generally poor polymerase substrates. While extensive synthetic efforts have yielded examples of HBAs with favorable substrate properties, their
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.