F9108
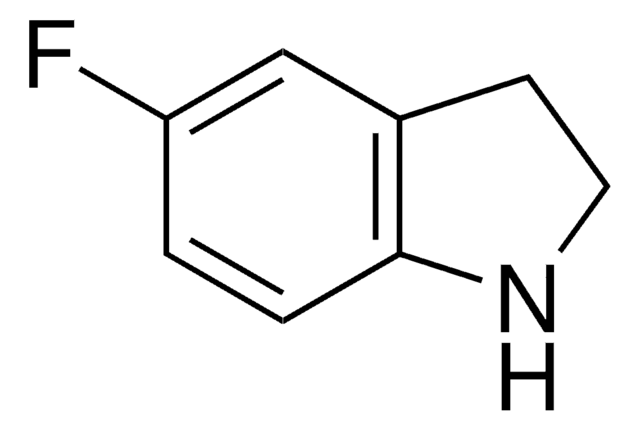
5-Fluor-indol
98%
Synonym(e):
NSC 88613
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C8H6FN
CAS-Nummer:
Molekulargewicht:
135.14
Beilstein:
112350
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
mp (Schmelzpunkt)
45-48 °C (lit.)
SMILES String
Fc1ccc2[nH]ccc2c1
InChI
1S/C8H6FN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H
InChIKey
ODFFPRGJZRXNHZ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Reactant for preparation of 5-HT6 receptor ligands
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for preparation of antitumor agents
- Reactant for preparation of antibacterial agents
- Reactant for preparation of immunosuppressive agents
- Reactant for preparation of Sodium-Dependent Glucose Co-transporter 2 (SGLT2) Inhibitors for the Management of Hyperglycemia in Diabetes
- Reactant for preparation of Myeloperoxidase Inhibitors
- Reactant for preparation of Potent Selective Serotonin Reuptake Inhibitors
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
230.0 °F - closed cup
Flammpunkt (°C)
110 °C - closed cup
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
A L Palombella et al.
Plant physiology, 117(2), 455-464 (1998-06-25)
We report the isolation of a Chlamydomonas reinhardtii cDNA that encodes the beta-subunit of tryptophan synthase (TSB). This cDNA was cloned by functional complementation of a trp-operon-deleted strain of Escherichia coli. Hybridization analysis indicated that the gene exists in a
Steffen P Graether et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 178(1), 65-71 (2005-10-04)
We show that it is feasible to use a minicoil for solid-state 19F 1H NMR experiments that has short pulse widths, good RF homogeneity, and excellent signal-to-noise for small samples while using low power amplifiers typical to liquid-state NMR. The
Nicholas A Magnus et al.
Organic letters, 12(16), 3700-3703 (2010-08-14)
A practical synthesis of the glycogen synthase kinase-3 (GSK3) inhibitor bisarylmaleimide 1 has been accomplished employing Pictet-Spengler methodology to access the indole 7-position in preparing the benzodiazepine tricyclic fragment. A seven-step linear sequence that starts with commercially available 5-fluoroindole 7
Peter B Crowley et al.
Chemical communications (Cambridge, England), 48(86), 10681-10683 (2012-09-25)
Fluorine-containing amino acids are valuable probes for the biophysical characterization of proteins. Current methods for (19)F-labeled protein production involve time-consuming genetic manipulation, compromised expression systems and expensive reagents. We show that Escherichia coli BL21, the workhorse of protein production, can
A J Barczak et al.
Genetics, 140(1), 303-313 (1995-05-01)
A study of the biochemical genetics of the Arabidopsis thaliana tryptophan synthase beta subunit was initiated by characterization of mutants resistant to the inhibitor 5-fluoroindole. Thirteen recessive mutations were recovered that are allelic to trp2-1, a mutation in the more
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.