M14935
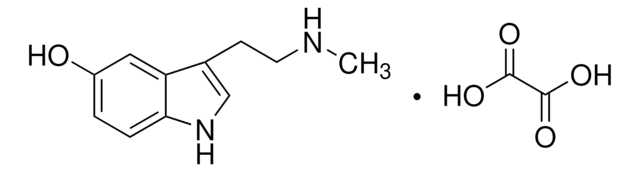
5-Methoxy-3-Indolessigsäure
98%
Synonym(e):
2-(5-Methoxy-3-indolyl)acetic acid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C11H11NO3
CAS-Nummer:
Molekulargewicht:
205.21
Beilstein:
187161
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
mp (Schmelzpunkt)
145-148 °C (dec.) (lit.)
SMILES String
COc1ccc2[nH]cc(CC(O)=O)c2c1
InChI
1S/C11H11NO3/c1-15-8-2-3-10-9(5-8)7(6-12-10)4-11(13)14/h2-3,5-6,12H,4H2,1H3,(H,13,14)
InChIKey
COCNDHOPIHDTHK-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
- Reactant for preparation of aryloxybenzothiazoles as inhibitors of NO production
- Reactant for preparation of transcription inhibitors of novel Gli1 as antitumor agents
- Reactant for preparation of indole derivatives as NR2B/NMDA receptor antagonists
- Reactant for preparation of indolyl esters and amides related to indomethacin as selective COX-2 inhibitors
- Reactant for preparation of quinoline salicylic acid series of P-selectin antagonists
- Reactant for preparation of prostaglandin D2 receptor antagonists
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
C W Tsang et al.
Biochemical and biophysical research communications, 209(3), 1132-1139 (1995-04-26)
The endogenous levels of 5-methoxyindole-3-acetic acid (5-MIAA) in quail pineal gland, retina and serum were determined by capillary column gas chromatography/mass spectrometry/selected ion monitoring using a deuterated internal standard and the N-pentafluoropropionyl-O-pentafluorobenzyl ester derivative. Diurnal rhythms of pineal and serum
T Ocal-Irez et al.
Brain research, 493(1), 1-7 (1989-07-24)
It has been suggested that the pineal gland has a specific role in the control of cyclic sexual activity in rats. One or more of the compounds isolated from this gland have been considered to be possible anti-fertility agents. In
P Li et al.
Journal of mass spectrometry : JMS, 31(11), 1228-1236 (1996-11-01)
A series of N-trifluoroacetyl/pentafluoropropionyl-O-trifluoroethyl/ pentafluoropropyl/heptafluorobutyl ester derivatives of 5-methoxyindole-3-acetic acid (5MIAA) were synthesized. Under electron-capture negative ionization conditions, the N-trifluoroacetyl derivatives were found to yield relatively abundant, analyte-specific M-. molecular ions and [M-HF]-., [M-HF-CF2CO]-. and [M-CF3CO]- fragment ions, while the
T I Sergeeva et al.
Voprosy onkologii, 33(10), 20-25 (1987-01-01)
Daily urinary excretion of 5-hydroxy-3-indoleacetic acid (5-HIAA) and 5-methoxyindole-3-acetic acid (5-MIAA) was studied by chromatography-mass-spectrometry in patients with cancer and healthy subjects. A considerable increase in urinary 5-MIAA-excretion was shown in patients with cancer of the stomach, rectum and lung.
Satoshi Furukawa et al.
The Journal of toxicological sciences, 30(3), 165-174 (2005-09-06)
Indole-3-acetic acid (IAA), a natural auxin, induces microencephaly in rats exposed to IAA during gestation days (Days) 12-14, corresponding to the early stage of cerebral cortex development. The purpose of this study was to examine the effects of 5 IAA
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.