Wichtige Dokumente
914924
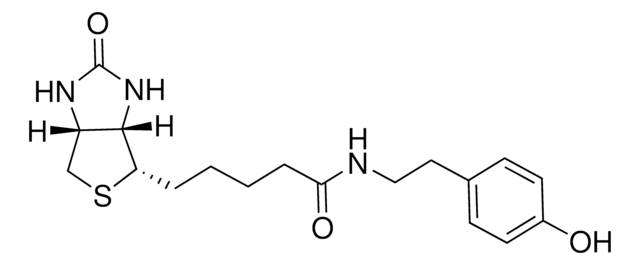
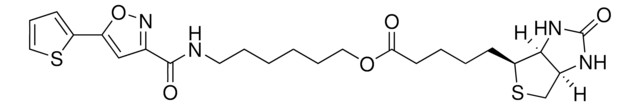
3-[2-N-(Biotinyl)aminoethyldithio]propanoic acid
≥95%
Synonym(e):
3-((2-(5-((3aS,4S,6aR)-2-Oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamido)ethyl)disulfaneyl)propanoic acid, Biotin-SS-COOH, Cleavable biotin linker
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
Form
powder
mp (Schmelzpunkt)
172-175 °C
Lagertemp.
−20°C
SMILES String
S1[C@H]([C@H]2NC(=O)N[C@H]2C1)CCCCC(=O)NCCSSCCC(=O)O
InChI
1S/C15H25N3O4S3/c19-12(16-6-8-25-24-7-5-13(20)21)4-2-1-3-11-14-10(9-23-11)17-15(22)18-14/h10-11,14H,1-9H2,(H,16,19)(H,20,21)(H2,17,18,22)/t10-,11-,14-/m0/s1
InChIKey
LUKYYZVIDAWYMZ-MJVIPROJSA-N
Anwendung
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Sonstige Hinweise
A Mechanism-Based ICAT Strategy for Comparing Relative Expression and Activity Levels of Glycosidases in Biological Systems
Dissociation-independent selection of high-affinity anti-hapten phage antibodies using cleavable biotin-conjugated haptens
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 914924-50MG | 4061842083893 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![Spiro[9H-fluorene-9,9′-[9H]xanthene]-2,7-diamine](/deepweb/assets/sigmaaldrich/product/structures/307/234/46c07f0c-9242-4c0b-8994-8121690da3c9/640/46c07f0c-9242-4c0b-8994-8121690da3c9.png)