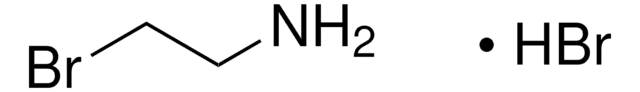
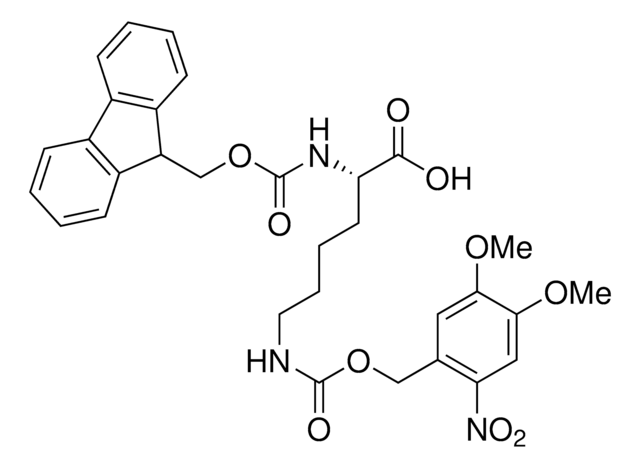
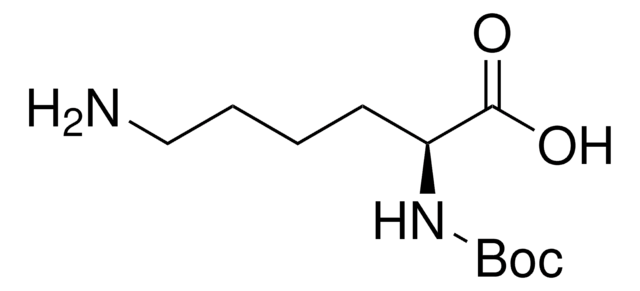
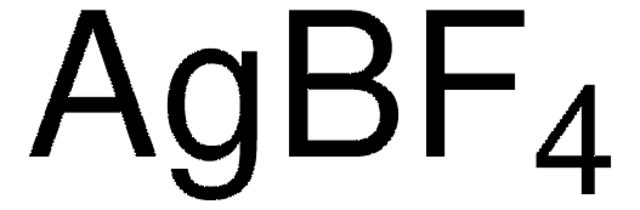Wichtige Dokumente
913588
O-(2-Nitrobenzyl)-L-tyrosine hydrochloride
≥95%
Synonym(e):
(S)-2-Amino-3-(4-((2-nitrobenzyl)oxy)phenyl)propanoic acid hydrochloride, NBY, ONBY, Photo-controlled amino acid, Photocaged amino acid, Photocleavable tyrosine derivative
About This Item
Empfohlene Produkte
Assay
≥95%
Form
powder
Verfügbarkeit
available only in USA
mp (Schmelzpunkt)
205 °C (decomp.)
Lagertemp.
2-8°C
InChI
1S/C16H16N2O5.ClH/c17-14(16(19)20)9-11-5-7-13(8-6-11)23-10-12-3-1-2-4-15(12)18(21)22;/h1-8,14H,9-10,17H2,(H,19,20);1H/t14-;/m0./s1
InChIKey
DRUCEARMIBXBOJ-UQKRIMTDSA-N
Anwendung
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Sonstige Hinweise
Time-resolved protein activation by proximal decaging in living systems
Crystal structure of a domain-swapped photoactivatable sfGFP variant provides evidence for GFP folding pathway
Light-control of the ultra-fast Gp41-1 split intein with preserved stability of a genetically encoded photo-caged amino acid in bacterial cells
Rapid and Inexpensive Evaluation of Nonstandard Amino Acid Incorporation in Escherichia coli
Ähnliches Produkt
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Self-react. C
Lagerklassenschlüssel
5.2 - Organic peroxides and self-reacting hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![N-[2-(Fmoc-amino)-ethyl]-Gly-O-tBu -hydrochlorid ≥98.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/641/926/3fedc773-b21f-4419-afd5-87e20df0156a/640/3fedc773-b21f-4419-afd5-87e20df0156a.png)

![[2,2′-Bipyridine]-6-carboxylic acid hydrochloride](/deepweb/assets/sigmaaldrich/product/structures/130/786/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d/640/b0e8142b-94b7-4abd-8a9a-0db3780a9f3d.png)