Alle Fotos(2)
Wichtige Dokumente
909335
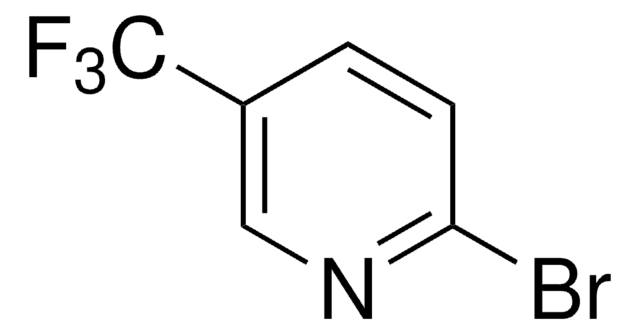
2-(2,4-Difluorophenyl)-5-(trifluoromethyl)pyridine
≥95%
Synonym(e):
dFCF3ppy
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C12H6F5N
CAS-Nummer:
Molekulargewicht:
259.17
MDL-Nummer:
UNSPSC-Code:
12352101
NACRES:
NA.22
Empfohlene Produkte
Assay
≥95%
Form
powder or crystals
Eignung der Reaktion
reaction type: Photocatalysis
reagent type: catalyst
mp (Schmelzpunkt)
59-64 °C
InChI
1S/C12H6F5N/c13-8-2-3-9(10(14)5-8)11-4-1-7(6-18-11)12(15,16)17/h1-6H
InChIKey
FMKQPMDFNYNYAG-UHFFFAOYSA-N
Anwendung
2-(2,4-Difluorophenyl)-5-(trifluoromethyl)pyridine is a ligand used for the preparation of Ir(III) photocatalysts.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Lot/Batch Number
Leider sind derzeit keine COAs für dieses Produkt online verfügbar.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kazimer L Skubi et al.
Journal of the American Chemical Society, 139(47), 17186-17192 (2017-11-01)
Stereochemical control of electronically excited states is a long-standing challenge in photochemical synthesis, and few catalytic systems that produce high enantioselectivities in triplet-state photoreactions are known. We report herein an exceptionally effective chiral photocatalyst that recruits prochiral quinolones using a
Thomas Rossolini et al.
Organic letters, 20(21), 6794-6798 (2018-10-24)
A visible-light-mediated photocatalytic umpolung synthesis of 1,3-diamines from in situ-generated imines and dehydroalanine derivatives is described. Pivoting on a key nucleophilic addition of photocatalytically generated α-amino radicals to electron-deficient alkenes, this three-component coupling reaction affords 1,3-diamines efficiently and diastereoselectively. The
Timothy M Monos et al.
Science (New York, N.Y.), 361(6409), 1369-1373 (2018-09-29)
Alkene aminoarylation with a single, bifunctional reagent is a concise synthetic strategy. We report a catalytic protocol for the addition of arylsulfonylacetamides across electron-rich alkenes with complete anti-Markovnikov regioselectivity and excellent diastereoselectivity to provide 2,2-diarylethylamines. In this process, single-electron alkene
John C Tellis et al.
Science (New York, N.Y.), 345(6195), 433-436 (2014-06-07)
The routine application of C(sp3)-hybridized nucleophiles in cross-coupling reactions remains an unsolved challenge in organic chemistry. The sluggish transmetalation rates observed for the preferred organoboron reagents in such transformations are a consequence of the two-electron mechanism underlying the standard catalytic
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)

![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)