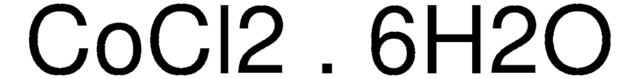769495
Cobalt(II)-Chlorid Hexahydrat
≥97%
Synonym(e):
Cobaltous chloride hexahydrate
About This Item
Empfohlene Produkte
Dampfdruck
40 mmHg ( 0 °C)
Qualitätsniveau
Assay
≥97%
97.0-102.0% (KT)
Form
solid
Anionenspuren
nitrate (NO3-): ≤0.01%
sulfate (SO42-): ≤0.007%
Kationenspuren
Fe: ≤0.005%
Ni: ≤0.15%
Pb: ≤0.002%
Zn: ≤0.05%
SMILES String
O.O.O.O.O.O.Cl[Co]Cl
InChI
1S/2ClH.Co.6H2O/h2*1H;;6*1H2/q;;+2;;;;;;/p-2
InChIKey
GFHNAMRJFCEERV-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- An additive to the electron transport layer (ETL) in perovskite solar cells to improve their performance, particularly by reducing energy losses and increasing the open-circuit voltage.
- A cobalt source for doping ZnO nanostructures. The incorporation of cobalt ions into the ZnO matrix is crucial for modifying its electronic and optical properties.
- A precursor to modify cobalt metal-organic framework (Co-MOF) derived carbon microspheres for application as anode materials in lithium-ion batteries.
Hinweis zur Analyse
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Dam. 1 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Lagerklassenschlüssel
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.