742945
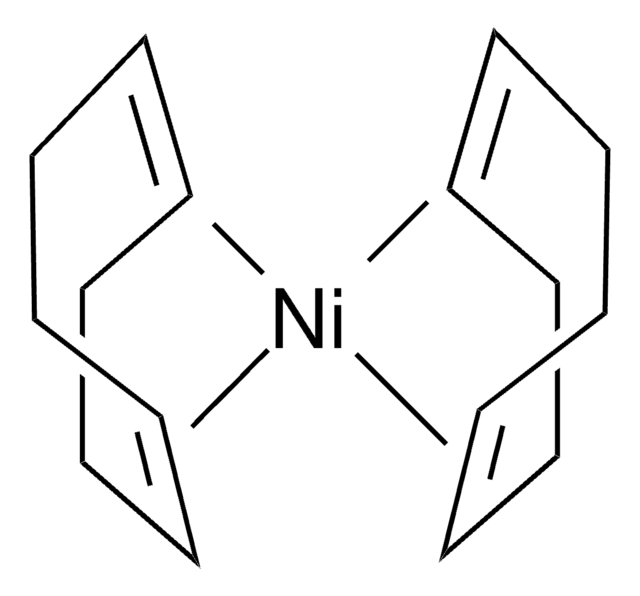
TurboBeads™ TEMPO
≥99%
Synonym(e):
Nanopartikel, magnetisch, TEMPO-modifiziert
About This Item
Empfohlene Produkte
Produktlinie
TurboBeads™
Assay
≥99%
Form
powder
Zusammensetzung
carbon content, ≤14 wt. %
Eignung der Reaktion
reaction type: solution phase peptide synthesis
reactivity: alcohol reactive
Kennzeichnungsgrad
≥0.1 mmol/g loading (TEMPO)
Magnetisierung
≥120 emu/g, mass saturation
Oberflächenbereich
≥15 m2/g
Durchschnittlicher Durchmesser
≤50 nm
Eignung
conforms to structure for Infrared spectrum
Anwendung
Verpackung
Hinweis zur Analyse
air-stability:
weight gain in air at 400°C >20 wt.%
weight gain in air at 100°C <3 wt.%
Rechtliche Hinweise
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.