513210
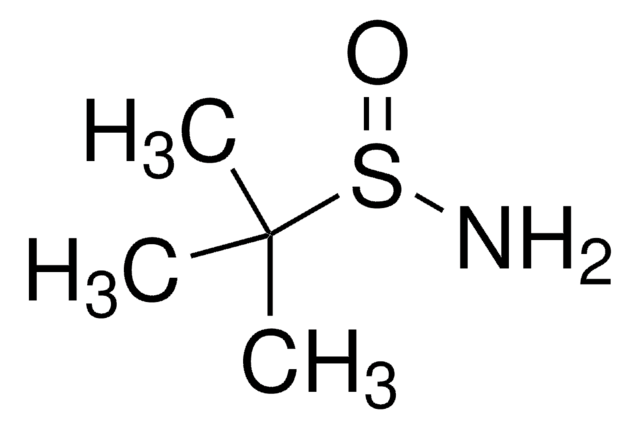
(S)-(−)-2-Methyl-2-propansulfinamid
97%
Synonym(e):
(S)-(-)-tert-Butanesulfinamide, (S)-(-)-tert-Butyl sulfinamide, (S)-2-Methyl-2-propanesulfinamide, (S)-tert-Butanesulfinamide, (S)-tert-Butylsulfinamide
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Optische Aktivität
[α]20/D −4.5°, c = 1 in chloroform
mp (Schmelzpunkt)
97-101 °C (lit.)
Lagertemp.
2-8°C
SMILES String
CC(C)(C)S(N)=O
InChI
1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3/t7-/m0/s1
InChIKey
CESUXLKAADQNTB-ZETCQYMHSA-N
Anwendung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
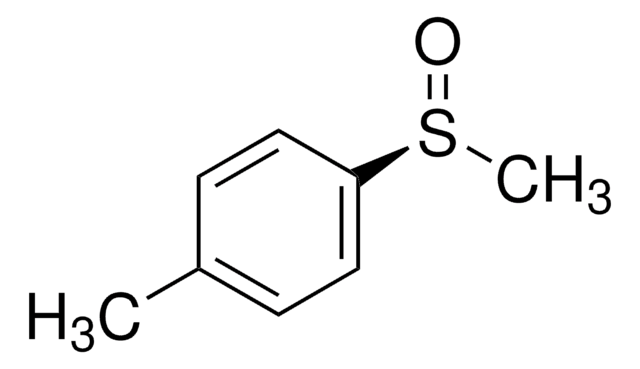
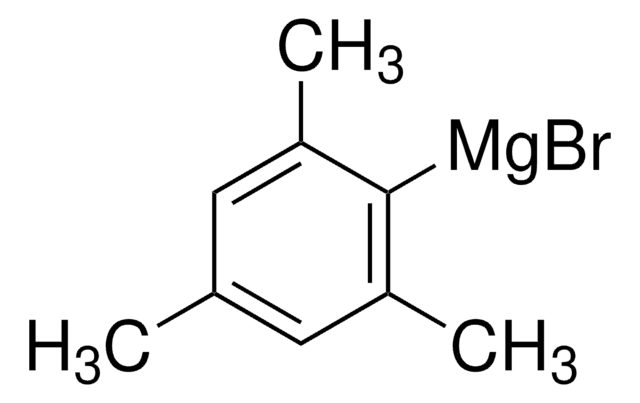
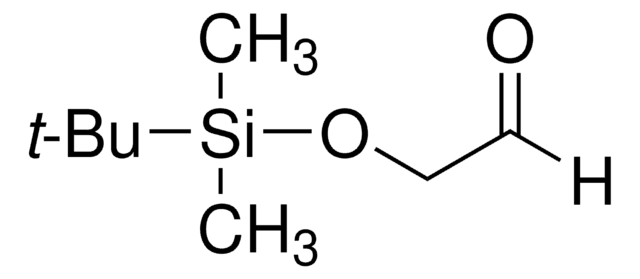
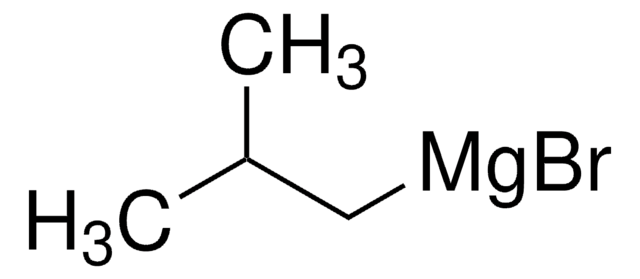
Kunden haben sich ebenfalls angesehen
Artikel
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Verwandter Inhalt
The Ellman group has participated in the development of a variety of C-H functionalization methods. An electron rich phosphine ligand has proven to be very useful for a variety of Rh(I)-catalyzed C-C bond forming reactions applicable to heterocycle synthesis as exemplified in the recent Science paper “Proton Donor Acidity Controls Selectivity in Nonaromatic Nitrogen Heterocycle Synthesis.” Another useful ligand developed for the highly functional group compatible direct arylation of nitrogen heterocycles is described in a 2008 J. Am. Chem. Soc. paper “Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation: Expanded Scope through Mechanistic Insight.” The Ellman group also developed the chiral amine reagent tert-Butanesulfinamide, which is extensively used in academics and industry for the asymmetric synthesis of amines. A comprehensive survey of tert-Butanesulfinamide methods and applications up through 2009 is provided in the 2010 Chemical Reviews article, “Synthesis and Applications of tert-Butanesulfinamide.”
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.