Wichtige Dokumente
440914
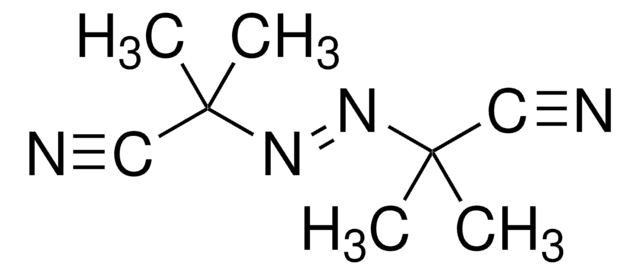
2,2′-Azo-bis(2-methylpropionamidin) -dihydrochlorid
powder or granules, 97%
Synonym(e):
α,α′-Azodiisobutyramidin -dihydrochlorid, AAPH
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
powder or granules
t1/2
10 hr(56 °C)
mp (Schmelzpunkt)
175-177 °C (lit.)
Löslichkeit
acetone, dioxane, methanol, ethanol, DMSO and water: soluble
SMILES String
Cl.Cl.CC(C)(\N=N\C(C)(C)C(N)=N)C(N)=N
InChI
1S/C8H18N6.2ClH/c1-7(2,5(9)10)13-14-8(3,4)6(11)12;;/h1-4H3,(H3,9,10)(H3,11,12);2*1H/b14-13+;;
InChIKey
LXEKPEMOWBOYRF-QDBORUFSSA-N
Verwandte Kategorien
Anwendung
Polymerisationsinhibitor für Acryl-, Vinyl- und Allylmonomere.
Leistungsmerkmale und Vorteile
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Self-heat. 1 - Skin Sens. 1
Lagerklassenschlüssel
4.2 - Pyrophoric and self-heating hazardous materials
WGK
WGK 1
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
We presents an article about a micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization. RAFT (Reversible Addition/Fragmentation Chain Transfer) polymerization is a reversible deactivation radical polymerization (RDRP) and one of the more versatile methods for providing living characteristics to radical polymerization.
We presents an article about Copper(I)-mediated Living Radical Polymerization in the Presence of Pyridylmethanimine Ligands, and the emergence of living radical polymerization mediated by transition metal catalysts in 1995, which was a seminal piece of work in the field of synthetic polymer chemistry.
Applying ARGET ATRP to the Growth of Polymer Brush Thin Films by Surface-initiated Polymerization
Protokolle
Monodisperse, surfactant-free polymer spheres for use as colloidal crystal templates can be easily obtained in reasonably large quantities. Typical synthesis methods for poly(methyl methacrylate) (PMMA) and poly(styrene) (PS) by emulsifier free emulsion polymerization are described below and yield spheres several hundred nanometers in diameter.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
An article about the typical procedures for polymerizing via ATRP, which demonstrates that in the following two procedures describe two ATRP polymerization reactions as performed by Prof. Dave Hadddleton′s research group at the University of Warwick.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 440914-25G | 4061835515516 |
| 440914-100G | 4061835563098 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.