Alle Fotos(3)
Wichtige Dokumente
431966
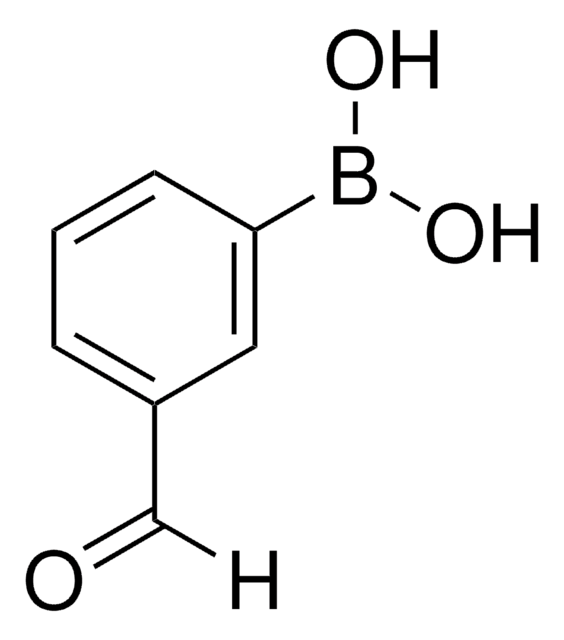
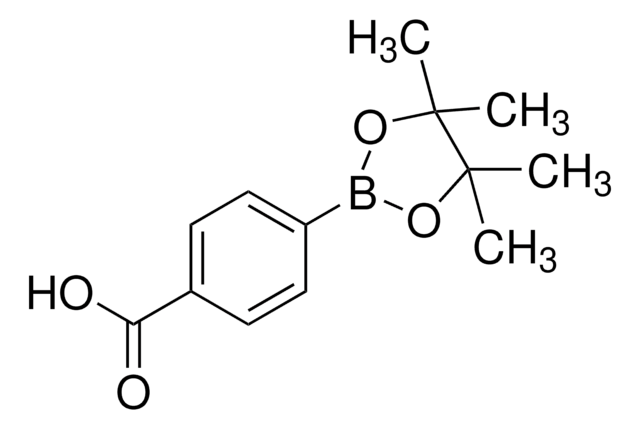
4-Formylphenylborsäure
≥95.0%
Synonym(e):
4-(Dihydroxyboryl)-benzaldehyd, 4-Borono-benzaldehyd
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Lineare Formel:
HCOC6H4B(OH)2
CAS-Nummer:
Molekulargewicht:
149.94
Beilstein:
3030770
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥95.0%
mp (Schmelzpunkt)
237-242 °C (lit.)
Funktionelle Gruppe
aldehyde
SMILES String
OB(O)c1ccc(C=O)cc1
InChI
1S/C7H7BO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5,10-11H
InChIKey
VXWBQOJISHAKKM-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
4-Formylphenylboronic acid is a substrate for Suzuki cross-coupling reactions and it can be used as a reagent for:
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Triethylamine-catalyzed three-component Hantzsch condensations.
- Copper-catalyzed nitrations.
- Oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta.
- Palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides.
- Palladium-catalyzed aerobic oxidative cross-coupling reactions.
- The synthesis of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells.
- The synthesis of a novel protein synthesis inhibitor active against Gram-positive bacteria.
- The Suzuki aryl-aryl coupling of the upper rim of hexahomotrioxacalix[3]arene.
- A rhodium-catalyzed cyclization, converting 1,5-enynes to cyclopentenes and spiro-cyclopentenes.
Sonstige Hinweise
Contains varying amounts of anhydride
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Skin Sens. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 1
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Synthesis of Some New 1,4-Dihydropyridine Derivatives through a Facile One-pot Hantzsch Condensation Catalyzed by Triethylamine
Chin. J. Chem., 30, 733-737 (2012)
Kunpeng Guo et al.
Organic letters, 14(9), 2214-2217 (2012-04-14)
This work identifies the dithiafulvenyl unit as an excellent electron donor for constructing D-π-A-type metal-free organic sensitizers of dye-sensitized solar cells (DSCs). Synthesized and tested are three sensitizers all with this donor and a cyanoacrylic acid acceptor but differing in
Tetrahedron, 62, 10321-10321 (2006)
Nora R Eibergen et al.
Chembiochem : a European journal of chemical biology, 13(4), 574-583 (2012-03-01)
In an effort to identify novel antibacterial chemotypes, we performed a whole-cell screen for inhibitors of Staphylococcus aureus growth and pursued those compounds with previously uncharacterized antibacterial activity. This process resulted in the identification of a benzothiazolium salt, ABTZ-1, that
Qi Huang et al.
ACS applied materials & interfaces, 11(17), 15861-15868 (2019-03-28)
Conjugated microporous polymers (CMPs) with high surface areas, tunable building blocks, and fully conjugated structures have found important applications in optoelectronics. Here, we report a new series of CMPs with tunable band gaps by introducing thiazolo[5,4- d] thiazole as the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.