Alle Fotos(2)
Wichtige Dokumente
412260
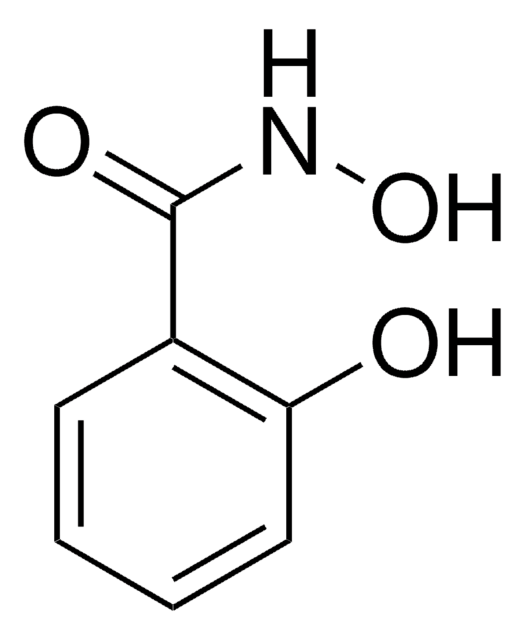
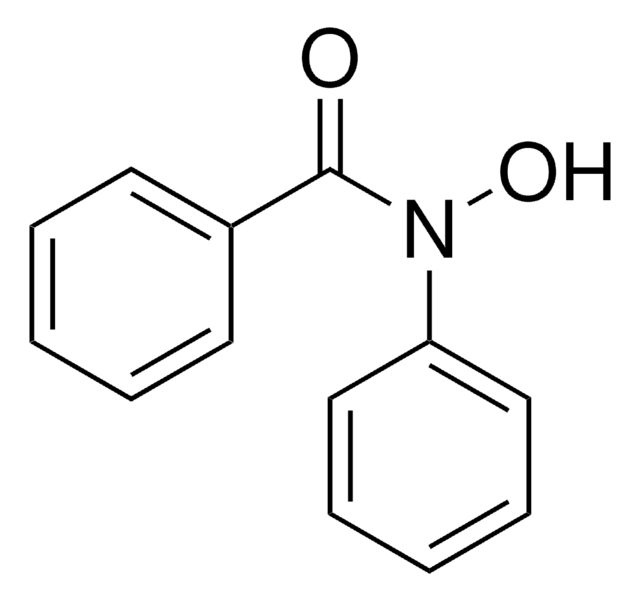
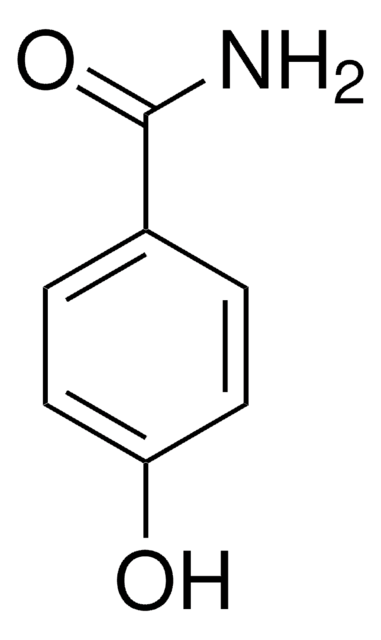
Benzhydroxamsäure
99%
Synonym(e):
N-Hydroxybenzamid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
C6H5CONHOH
CAS-Nummer:
Molekulargewicht:
137.14
Beilstein:
1907585
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
mp (Schmelzpunkt)
126-130 °C (lit.)
Löslichkeit
1 M NaOH: soluble 50 mg/mL, clear, colorless
Funktionelle Gruppe
amine
phenyl
Lagertemp.
2-8°C
SMILES String
ONC(=O)c1ccccc1
InChI
1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9)
InChIKey
VDEUYMSGMPQMIK-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Benzhydroxamic acid (BHA) reacts with BiPh3 or Bi(O(t)Bu)3 to afford novel mono- and di-anionic hydroxamato complexes, having anti-bacterial activity against Helicobacter pylori. Three-dimensional structure of recombinant horseradish peroxidase-BHA complex has been studied.
Anwendung
Benzhydroxamic acid may be employed for the Pd-catalyzed synthesis of benzisoxazolones.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
A Henriksen et al.
Biochemistry, 37(22), 8054-8060 (1998-06-12)
The three-dimensional structure of recombinant horseradish peroxidase in complex with BHA (benzhydroxamic acid) is the first structure of a peroxidase-substrate complex demonstrating the existence of an aromatic binding pocket. The crystal structure of the peroxidase-substrate complex has been determined to
Swetlana Gez et al.
Inorganic chemistry, 44(8), 2934-2943 (2005-04-12)
A new family of relatively stable Cr(V) complexes, [Cr(V)O(L)(2)](-) (LH(2) = RC(O)NHOH, R = Me, Ph, 2-HO-Ph, or HONHC(O)(CH(2))(6)), has been obtained by the reactions of hydroxamic acids with Cr(VI) in polar aprotic solvents. Similar reactions in aqueous solutions led
Fernando Ruy et al.
Journal of bioenergetics and biomembranes, 38(2), 129-135 (2006-10-21)
Candida parapsilosis mitochondria contain three respiratory chains: the classical respiratory chain (CRC), a secondary parallel chain (PAR) and an "alternative" oxidative pathway (AOX). We report here the existence of similar pathways in C. albicans. To observe the capacity of each
Florian Thaler et al.
Journal of medicinal chemistry, 53(2), 822-839 (2009-12-19)
The histone deacetylases (HDACs) are able to regulate gene expression, and histone deacetylase inhibitors (HDACi) emerged as a new class of agents in the treatment of cancer as well as other human disorders such as neurodegenerative diseases. In the present
Henrik R Hallingbäck et al.
Biochemistry, 45(9), 2940-2950 (2006-03-01)
The oxidation of melatonin by the mammalian myeloperoxidase (MPO) provides protection against the damaging effects of reactive oxygen species. Indole derivatives, such as melatonin and serotonin, are also substrates of the plant horseradish peroxidase (HRP), but this enzyme exhibits remarkable
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.