392847
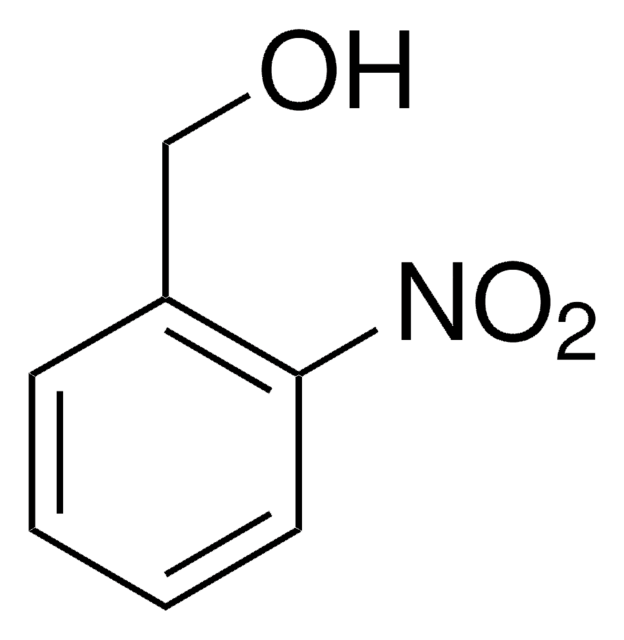
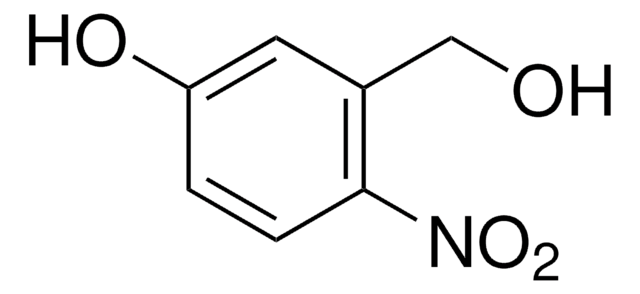
4,5-Dimethoxy-2-nitrobenzylalkohol
98%
Synonym(e):
6-Nitroveratrylalkohol
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
O2NC6H2(OCH3)2CH2OH
CAS-Nummer:
Molekulargewicht:
213.19
Beilstein:
1880093
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
mp (Schmelzpunkt)
145-148 °C (lit.)
Funktionelle Gruppe
hydroxyl
nitro
SMILES String
COc1cc(CO)c(cc1OC)[N+]([O-])=O
InChI
1S/C9H11NO5/c1-14-8-3-6(5-11)7(10(12)13)4-9(8)15-2/h3-4,11H,5H2,1-2H3
InChIKey
WBSCOJBVYHQOFB-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-Nitroveratryl Alcohol) is 2-nitrobenzyl alcohol derivative. It has been reported to be one of the oxidation products of veratryl (3,4-dimethoxybenzyl) alcohol by lignin peroxidase (isolated from Phanerochaete chrysosporium).
Anwendung
4,5-Dimethoxy-2-nitrobenzyl alcohol (6-nitroveratryl alcohol) is suitable reagent used in the synthesis of 4,5-dimethoxy-2-nitrobenzyl methacrylate, a photolabile monomer and 2-(4-((4-(4,5-dimethoxy-2-nitrobenzyloxy)phenyl)cyclohexylidene)methyl)phenoxy)-N,N-dimethylethanamine, a caged cyclofen-OH ligand.
It may be used in the synthesis of the following:
It may be used in the synthesis of the following:
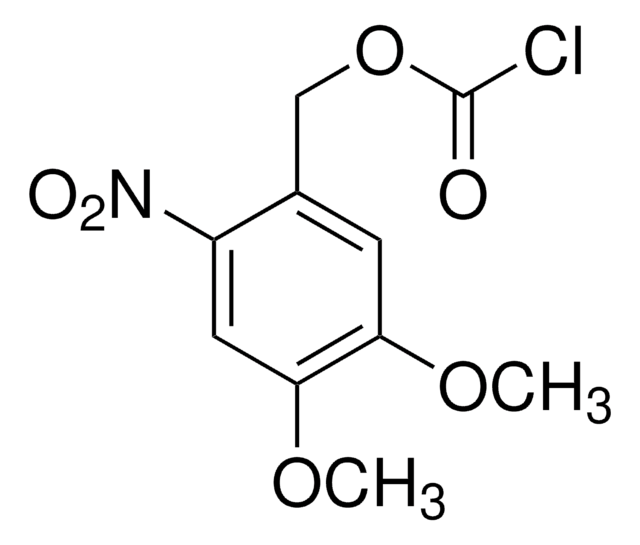
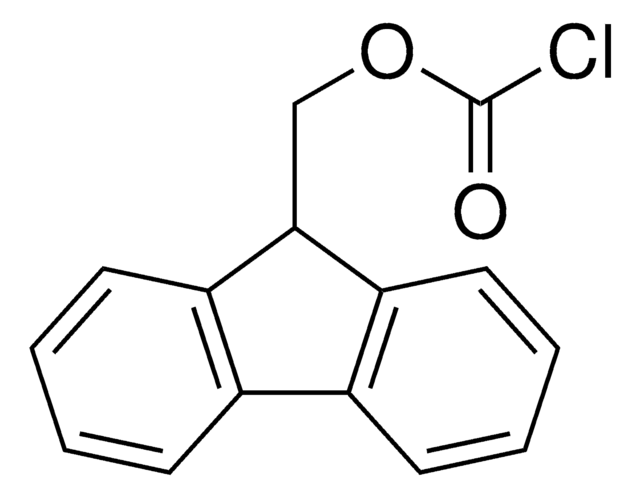
- 1-[[(chlorocarbonyl)oxy]methyl]-4,5-dimethoxy-2-nitrobenzene
- bis(4,5-dimethoxy-2-nitrophenyl)ethylene glycol, a photolabile protecting group
- optically-sensitive monomer
- nitroveratryl (NV) protected α-hydroxyacetic acid (αG) (NV-αG-OH), required in the preparation of nitroveratryl (NV) protected cyanomethyl (CM) ester of α-hydroxyacetic acid (αG) (NV-αG-CM)
- 4,5-dimethoxy-2-nitrobenzyl p-nitro-phenylcarbonate
- 6-nitroveratryloxycarbonyl chloride (NVOCCl), a reagent used in the protection of amino function in amino sugars
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Andrew A Brown et al.
Langmuir : the ACS journal of surfaces and colloids, 25(3), 1744-1749 (2009-01-10)
The use of photolabile protecting groups (PGs) as a means to create latent hydrophilic surfaces is presented. Naturally hydrophobic PGs, based on o-nitrobenzyl chemistry, are used on polymer side chains, poised for cleavage upon exposure to UV light. Removal of
Peptide backbone mutagenesis of putative gating hinges in a potassium ion channel.
Yasuo Nagaoka et al.
Chembiochem : a European journal of chemical biology, 9(11), 1725-1728 (2008-06-11)
Novel photosensitive polyimide precursor based on polyisoimide using an amine photogenerator.
Mochizuki A, et al.
Macromolecules, 28(1), 365-369 (1995)
Photosensitive protecting groups of amino sugars and their use in glycoside synthesis. 2-nitrobenzyloxycarbonylamino and 6-nitroveratryloxycarbonylamino derivatives.
Amit B, et al.
The Journal of Organic Chemistry, 39(2), 192-196 (1974)
Nadezda Fomina et al.
Journal of the American Chemical Society, 132(28), 9540-9542 (2010-06-24)
A new light-sensitive polymer containing multiple light-sensitive triggering groups along the backbone and incorporating a quinone-methide self-immolative moiety was developed and formulated into nanoparticles encapsulating a model pharmaceutical Nile Red. Triggered burst release of the payload upon irradiation and subsequent
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.