390704
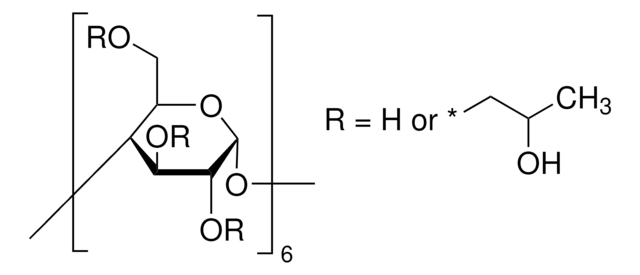
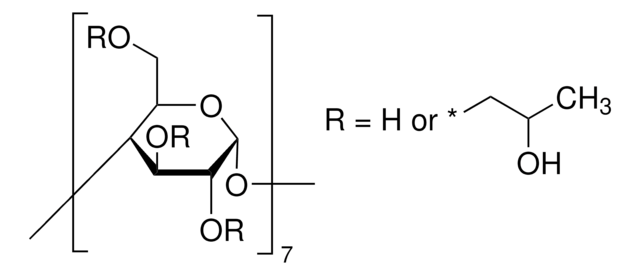
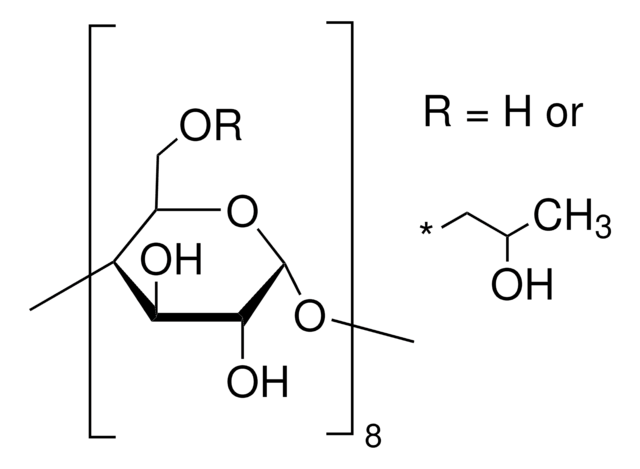
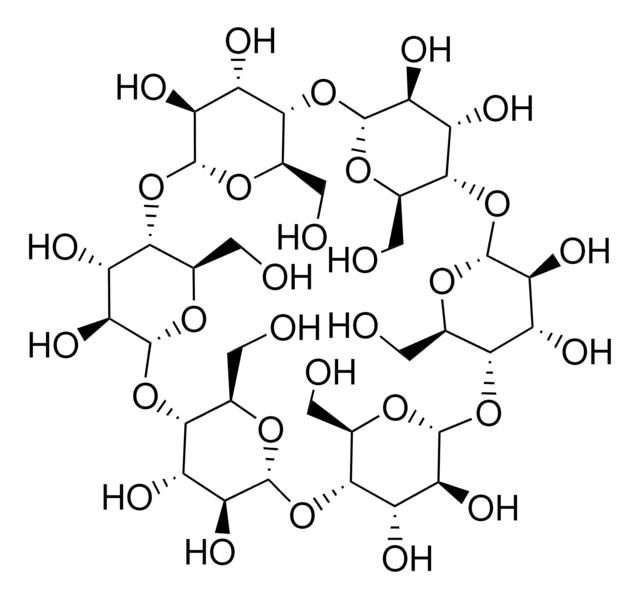
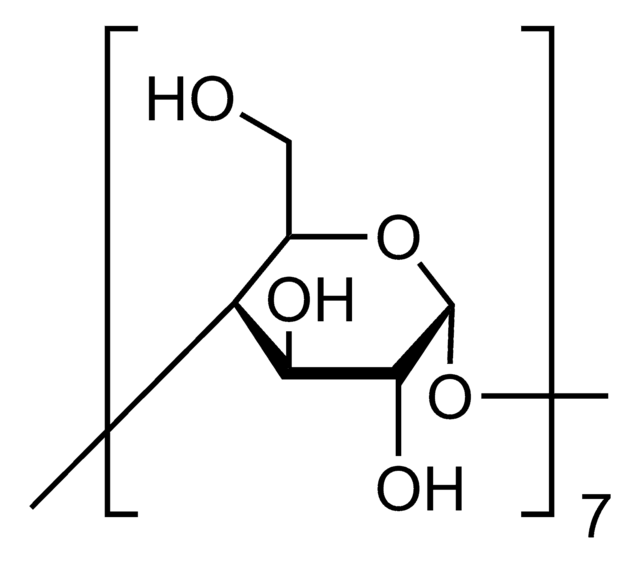
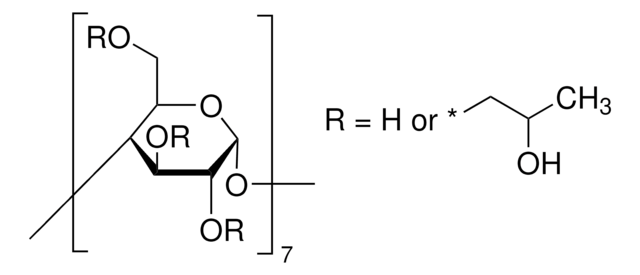
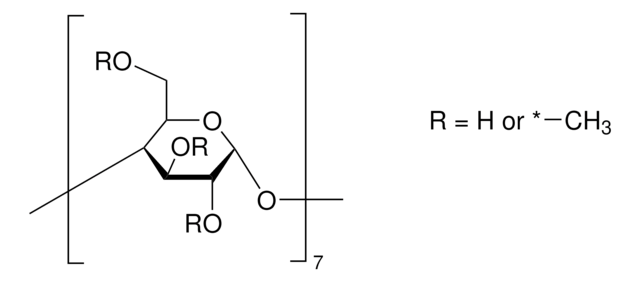
(2-Hydroxypropyl)-γ-cyclodextrin
extent of labeling: 0.6 molar substitution
Synonym(e):
HGC
About This Item
Empfohlene Produkte
Form
powder
Optische Aktivität
[α]20/D +145°, c = 1 in H2O
Mol-Gew.
average Mw ~1,580
Kennzeichnungsgrad
0.6 molar substitution
InChI
1S/C51H88O38/c1-14(56)8-73-11-21-42-29(64)36(71)50(81-21)85-40-19(6-54)79-48(34(69)27(40)62)89-44-23(13-75-10-16(3)58)82-51(37(72)30(44)65)86-41-20(7-55)78-47(33(68)26(41)61)88-43-22(12-74-9-15(2)57)80-49(35(70)28(43)63)84-39-18(5-53)76-45(31(66)24(39)59)83-38-17(4-52)77-46(87-42)32(67)25(38)60/h14-72H,4-13H2,1-3H3/t14?,15?,16?,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36-,37-,38-,39-,40-,41-,42-,43-,44-,45-,46-,47-,48-,49-,50-,51-/m0/s1
InChIKey
ODLHGICHYURWBS-RYJYQAAZSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- As a mobile phase additive in the study of the host-guest interaction with organic low molecular mass compounds prior to their quantification using reversed phase-high performance liquid chromatography (RP-HPLC) technique.
- As a chiral surfactant for the analysis of econazole by micellar electrokinetic chromatography (MEKC).
- As an analytical standard for the determination of the analyte in biological samples by HPLC.
- As a chiral selector for the identification of propiconazole by MEKC.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.