Alle Fotos(2)
Wichtige Dokumente
389439
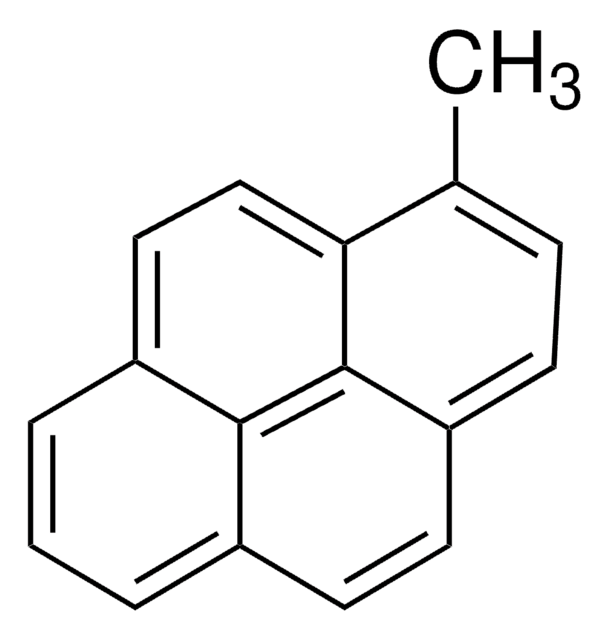
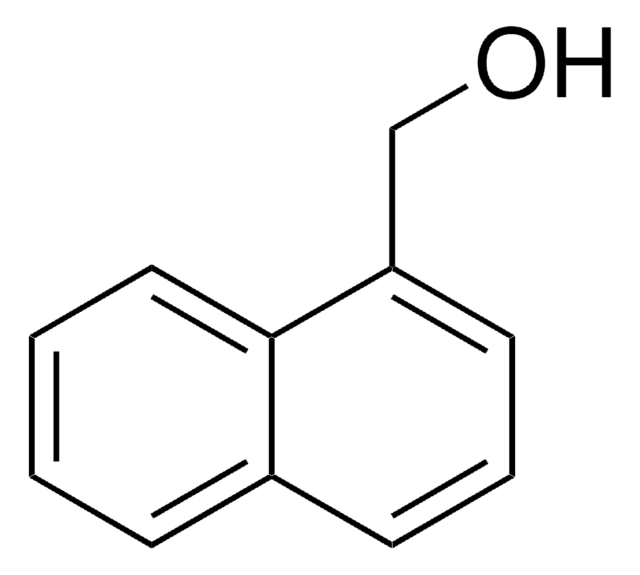
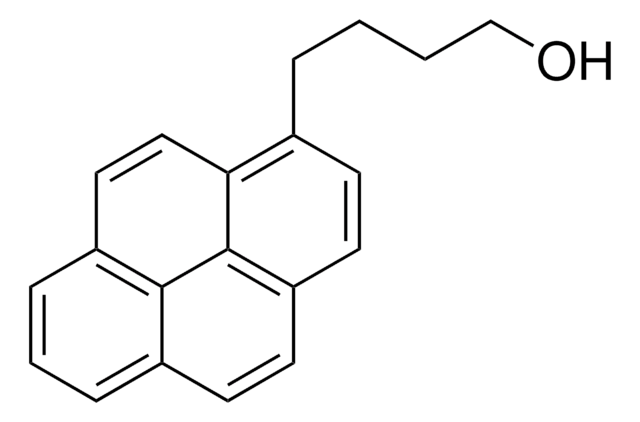
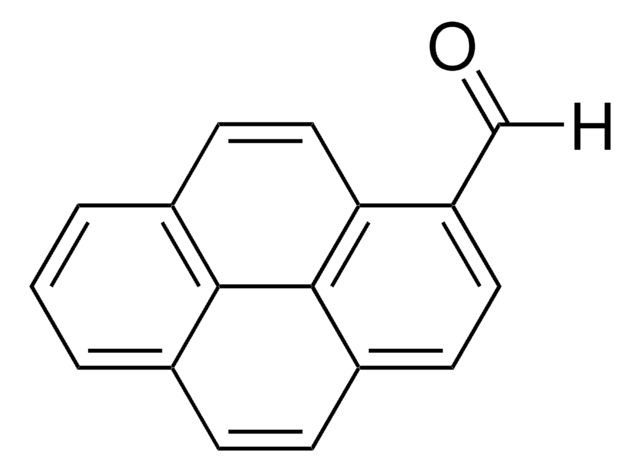
1-Pyrenmethanol
98%
Synonym(e):
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C17H12O
CAS-Nummer:
Molekulargewicht:
232.28
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
Form
solid
mp (Schmelzpunkt)
123-126 °C (lit.)
Funktionelle Gruppe
hydroxyl
SMILES String
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
InChIKey
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Anwendung
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
H Glatt et al.
Carcinogenesis, 14(4), 599-602 (1993-04-01)
1-Hydroxymethylpyrene (HMP), a primary benzylic alcohol, and 4H-cyclopenta[def]chrysen-4-ol (OH-CPC), a secondary benzylic alcohol, were investigated for mutagenicity in Salmonella typhimurium (reversion of the his- strain TA98) in the presence of various xenobiotic-metabolizing systems. In the direct test, HMP was inactive
Encapsulation of functional moieties within branched star polymers: effect of chain length and solvent on site isolation.
Hecht S, et al.
Journal of the American Chemical Society, 123(1), 18-25 (2001)
Walter Meinl et al.
Pharmacogenetics, 12(9), 677-689 (2002-12-05)
Various enzymatically formed sulfuric acid esters are chemically reactive and mutagenic. This metabolic activation pathway is not detected in standard in-vitro mutagenicity test systems. We describe the construction of Salmonella typhimurium TA1538-derived strains expressing alloenzymes *1, *2, *3, *5, *6
Highly efficient oxidation of alcohols to carbonyl compounds in the presence of molecular oxygen using a novel heterogeneous ruthenium catalyst.
Ji H, et al.
Tetrahedron Letters, 43(40), 7179-7183 (2002)
H Glatt et al.
Chemico-biological interactions, 109(1-3), 195-219 (1998-05-05)
Sulfation is a common final step in the biotransformation of xenobiotics and is traditionally associated with inactivation. However, the sulfate group is electron-withdrawing and may be cleaved off heterolytically in some molecules leading to electrophilic cations which may form adducts
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)