262218
Pentafluornitrobenzol
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
C6F5NO2
CAS-Nummer:
Molekulargewicht:
213.06
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
liquid
Brechungsindex
n20/D 1.447 (lit.)
bp
158-161 °C (lit.)
Dichte
1.656 g/mL at 25 °C (lit.)
Funktionelle Gruppe
fluoro
nitro
SMILES String
[O-][N+](=O)c1c(F)c(F)c(F)c(F)c1F
InChI
1S/C6F5NO2/c7-1-2(8)4(10)6(12(13)14)5(11)3(1)9
InChIKey
INUOFQAJCYUOJR-UHFFFAOYSA-N
Allgemeine Beschreibung
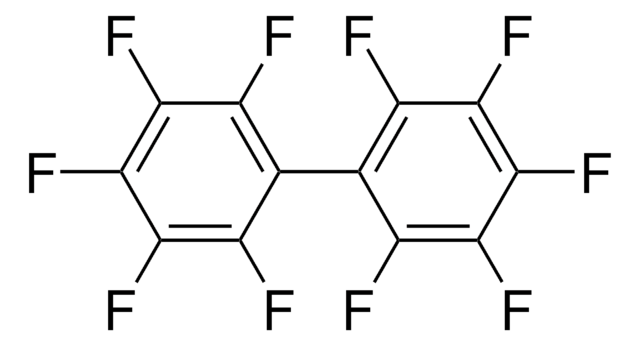
Electron attachment to pentafluoronitrobenzene has been studied in the energy range 0-16eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. The electroreduction of pentafluoronitrobenzene in dimethylformamide solution results in the formation of the dimer, octafluoro-4,4′-dinitro-biphenyl.
Anwendung
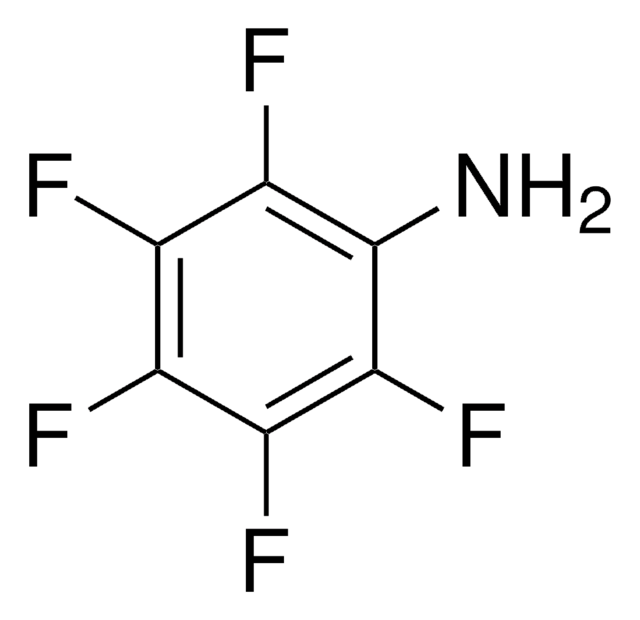
Pentafluoronitrobenzene has been used in the preparation of p-azidotetrafluoroaniline, a new photoaffinity reagent.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
195.8 °F - closed cup
Flammpunkt (°C)
91 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Judith Langer et al.
Physical chemistry chemical physics : PCCP, 10(11), 1523-1531 (2008-03-11)
Electron attachment to pentafluorobenzonitrile (C(6)F(5)CN) and pentafluoronitrobenzene (C(6)F(5)NO(2)) is studied in the energy range 0-16 eV by means of a crossed electron-molecular beam experiment with mass spectrometric detection of the anions. We find that pentafluoronitrobenzene exclusively generates fragment anions via
Voltammetry under high mass transport conditions. The application of the high speed channel electrode to the reduction of pentafluoronitrobenzene.
Coles BA, et al.
Journal of Electroanalytical Chemistry, 411(1), 121-127 (1996)
K A Chehade et al.
The Journal of organic chemistry, 65(16), 4949-4953 (2000-08-24)
p-Azidotetrafluoroaniline (1) was synthesized in 65-73% yield by two different methods employing a stable carbamate intermediate. The first method trapped the intermediate isocyanate generated via a modified Curtius rearrangement with 2-methyl-2-propanol or 2-(trimethylsilyl)ethanol to form the stable carbamates 2d and
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.