Alle Fotos(2)
Wichtige Dokumente
261394
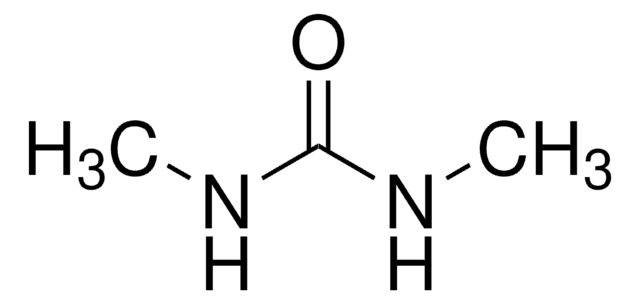
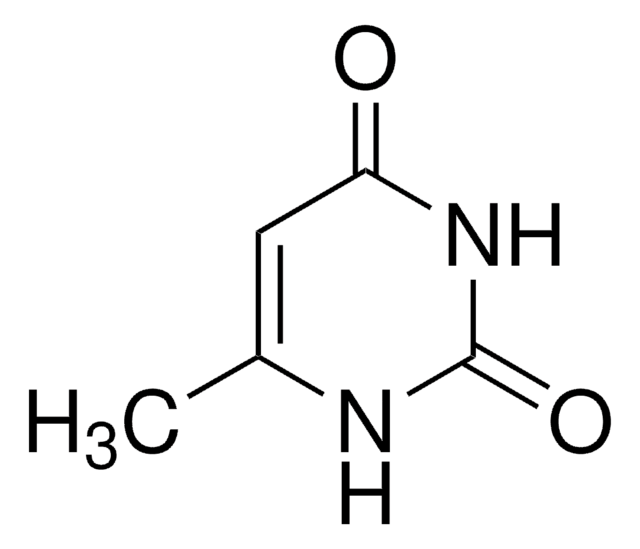
N,N-Dimethylharnstoff
99%
Synonym(e):
N,N-Dimethylurea
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
(CH3)2NCONH2
CAS-Nummer:
Molekulargewicht:
88.11
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
99%
Form
solid
mp (Schmelzpunkt)
178-183 °C (lit.)
Löslichkeit
water: soluble 5%, clear, colorless
Funktionelle Gruppe
amine
SMILES String
CN(C)C(N)=O
InChI
1S/C3H8N2O/c1-5(2)3(4)6/h1-2H3,(H2,4,6)
InChIKey
YBBLOADPFWKNGS-UHFFFAOYSA-N
Angaben zum Gen
human ... EPHX2(2053)
mouse ... Ephx2(13850)
Allgemeine Beschreibung
Nonlinear optical properties of 1,1-dimethylurea (N,N′ dimethylurea), have been evaluated through second-harmonic generation.
Anwendung
1,1-Dimethylurea (N,N-dimethylurea) has been used in the Dowex-50W ion exchange resin-promoted synthesis of N,N′-disubstituted-4-aryl-3,4-dihydropyrimidinones.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
S Sandler et al.
Diabetologia, 23(4), 374-378 (1982-10-01)
The protective effect on streptozotocin-induced diabetes of dimethyl urea, a hydroxyl radical scavenger, has been evaluated in vivo and in vitro. Pretreatment with dimethyl urea before a single diabetogenic dose of streptozotocin partially protected NMRI mice from hyperglycaemia, whereas the
Nonlinear optical properties of N, N' dimethylurea.
Halbout JM, et al.
Applied Physics Letters, 37(10), 864-866 (2008)
G L Wilson et al.
Diabetologia, 27(6), 587-591 (1984-12-01)
In studies to evaluate possible inhibitors of the B-cell toxin, streptozotocin, the superoxide scavenger, superoxide dismutase, did not prevent or reduce the toxic effects of streptozotocin as determined by loss of insulin secretion from rat pancreatic B cells in monolayer
G P Meshram et al.
Mutation research, 279(4), 275-280 (1992-06-16)
Methyl isocyanate (MIC) in aqueous solution forms methylamine (MA) and N,N'-dimethylurea (DMU). MA in buffered system further converts into its salt form, methylamine hydrochloride (MAH). Therefore, MAH and DMU were evaluated for their mutagenic activity in the in vitro Ames
W J Caspary et al.
Mutation research, 174(4), 285-293 (1986-08-01)
Methylisocyanate (MIC) induced mutagenic responses in the absence of exogenous activation in the mouse lymphoma cell forward mutation assay at concentrations as low as 8-24 microM. MIC produced predominantly small mutant colonies, suggesting the possibility of clastogenic activity. The intermediate
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.