About This Item
Empfohlene Produkte
Assay
98%
Optische Aktivität
[α]23/D +42.8°, c = 1 in pyridine
mp (Schmelzpunkt)
232 °C (dec.) (lit.)
SMILES String
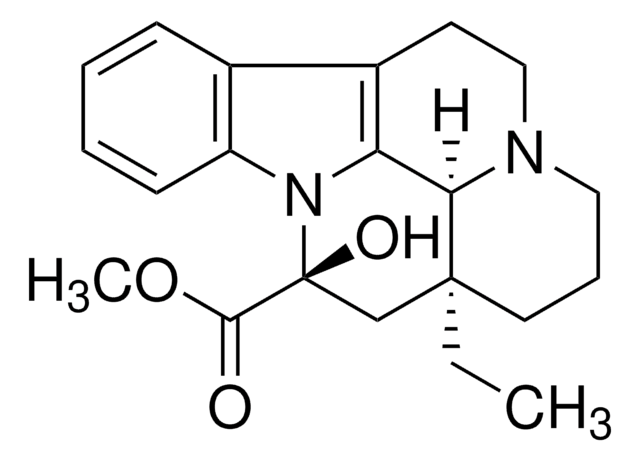
CC[C@@]12CCCN3CCc4c(C13)n(c5ccccc45)[C@](O)(C2)C(=O)OC
InChI
1S/C21H26N2O3/c1-3-20-10-6-11-22-12-9-15-14-7-4-5-8-16(14)23(17(15)18(20)22)21(25,13-20)19(24)26-2/h4-5,7-8,18,25H,3,6,9-13H2,1-2H3/t18-,20+,21+/m1/s1
InChIKey
RXPRRQLKFXBCSJ-GIVPXCGWSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
- Vincamine acid, which is employed as a precursor in the synthesis of vinpocetine by dehydration and esterification using sulfuric acid.
- Apovincamine using iron(III) perchlorate.
- (-)-Criocerine via one-step iodination reaction.
Verpackung
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.