Alle Fotos(1)
Wichtige Dokumente
183172
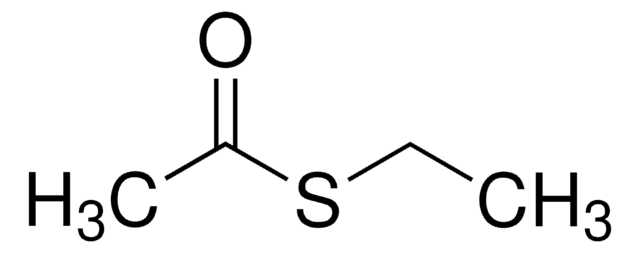
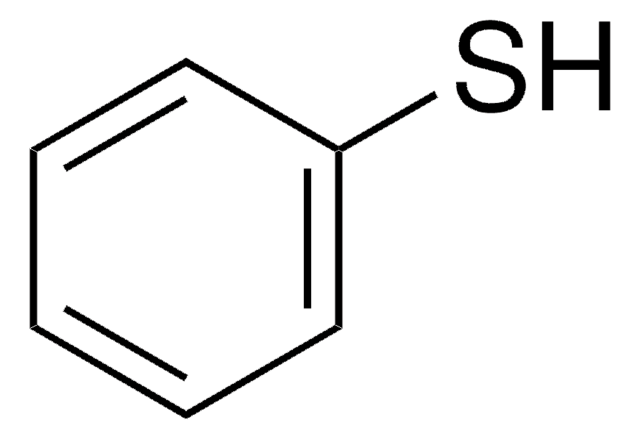
S-Phenylthioacetat
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
CH3COSC6H5
CAS-Nummer:
Molekulargewicht:
152.21
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
Form
liquid
Brechungsindex
n20/D 1.57 (lit.)
bp
99-100 °C/6 mmHg (lit.)
Dichte
1.124 g/mL at 25 °C (lit.)
Funktionelle Gruppe
thioester
Lagertemp.
2-8°C
SMILES String
CC(=O)Sc1ccccc1
InChI
1S/C8H8OS/c1-7(9)10-8-5-3-2-4-6-8/h2-6H,1H3
InChIKey
WBISVCLTLBMTDS-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
S-Phenyl thioacetate was used as a substrate to measure the esterase activity.
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 2
Flammpunkt (°F)
174.2 °F - closed cup
Flammpunkt (°C)
79 °C - closed cup
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Yu Yuan et al.
Journal of the American Chemical Society, 131(15), 5432-5437 (2009-04-22)
Described herein is the chemical synthesis of the Cys(29)-Gly(77) glycopeptide domain (22) of erythropoietin. Our initial ligation strategy targeted a C --> N termini condensation between glycopeptide 3 and peptide 4. However, the reaction was hindered by the "unattainable" reactivity
K Lorentz et al.
Clinica chimica acta; international journal of clinical chemistry, 308(1-2), 69-78 (2001-06-20)
Arylesterase (EC 3.1.1.2) activity in serum was specifically measured using thiophenyl acetate in a mechanized assay at 37 degrees C with 4-bromophenylboronic acid as inhibitor of cholinesterase and hexacyanoferrate-III as indicator. The systematic development of a routine method, apparent limitations
Anita Bosak et al.
Molecules (Basel, Switzerland), 25(1) (2020-01-18)
Mammalian paraoxonase-1 hydrolyses a very broad spectrum of esters such as certain drugs and xenobiotics. The aim of this study was to determine whether carbamates influence the activity of recombinant PON1 (rePON1). Carbamates were selected having a variety of applications:
D I Draganov et al.
The Journal of biological chemistry, 275(43), 33435-33442 (2000-08-10)
The paraoxonase gene family contains at least three members: PON1, PON2, and PON3. The physiological roles of the corresponding gene products are still uncertain. Until recently, only the serum paraoxonase/arylesterase (PON1) had been purified and characterized. Here we report the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.