Wichtige Dokumente
178756
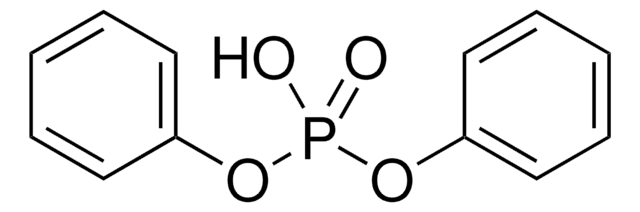
Diphenylphosphorylazid
97%
Synonym(e):
DPPA, Diphenylphosphoryl azid
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
liquid
Eignung der Reaktion
reaction type: click chemistry
Brechungsindex
n20/D 1.551 (lit.)
bp
157 °C/0.17 mmHg (lit.)
Dichte
1.277 g/mL at 25 °C (lit.)
Funktionelle Gruppe
azide
Lagertemp.
2-8°C
SMILES String
[N-]=[N+]=NP(=O)(Oc1ccccc1)Oc2ccccc2
InChI
1S/C12H10N3O3P/c13-14-15-19(16,17-11-7-3-1-4-8-11)18-12-9-5-2-6-10-12/h1-10H
InChIKey
SORGEQQSQGNZFI-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
Reagent for synthesis of oligosaccharides linked with carbamate and urea bonds utilizing modified Curtis rearrangement
Signalwort
Danger
Gefahreneinstufungen
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flammpunkt (°F)
233.6 °F - closed cup
Flammpunkt (°C)
112 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
The generation of an acid chloride is an obvious way to activate the carboxy group for amide bond formation. However, practical application of acid chlorides in peptide synthesis is restricted, because they are prone to side reactions and racemization.
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![1,8-Diazabicyclo[5.4.0]undec-7-en (1,5-5) 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)


![1,4-Diazabicyclo[2.2.2]octan ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)