178098
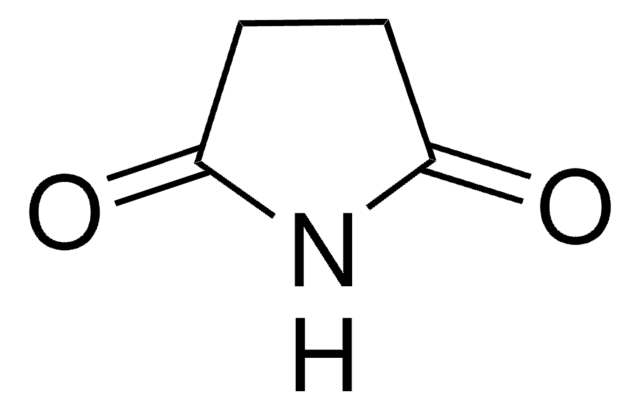
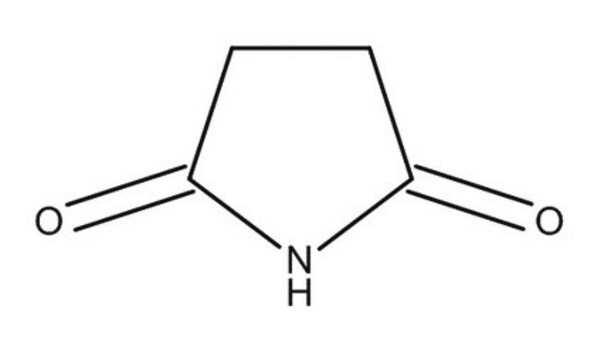
Glutarimid
98%
Synonym(e):
2,6-Piperidinedione, NSC 58190
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C5H7NO2
CAS-Nummer:
Molekulargewicht:
113.11
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Assay
98%
Form
solid
mp (Schmelzpunkt)
155-157 °C (lit.)
SMILES String
O=C1CCCC(=O)N1
InChI
1S/C5H7NO2/c7-4-2-1-3-5(8)6-4/h1-3H2,(H,6,7,8)
InChIKey
KNCYXPMJDCCGSJ-UHFFFAOYSA-N
Allgemeine Beschreibung
A glutarimide antibiotic, 9-methylstreptimidone, shows antiviral, antitumor and antifungal activities.
Anwendung
Reactant for:
Thionations
Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture
Synthesis of β-adrenoceptor ligands
Enantioselective synthesis of securinega alkaloids
Intramolecular amidocyclopropanation reactions
Synthesis of alpha-fluoro-alpha amino amides
Thionations
Biocatalytic asymmetric synthesis of chiral amines from ketones applied to sitagliptin manufacture
Synthesis of β-adrenoceptor ligands
Enantioselective synthesis of securinega alkaloids
Intramolecular amidocyclopropanation reactions
Synthesis of alpha-fluoro-alpha amino amides
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Alexander A Bisset et al.
Chemical communications (Cambridge, England), 48(98), 11978-11980 (2012-11-07)
The synthesis of (3E)-1-benzyl-3-[(2-oxopyridin-1(2H)-yl)methylidene]piperidine-2,6-dione 5 from N-benzylglutarimide was achieved in three steps. The asymmetric hydrogenation of 4 gave either the product of partial reduction (10) or full reduction (13), depending on the catalyst which was employed, in high ee in
Deevi Basavaiah et al.
Organic & biomolecular chemistry, 6(6), 1034-1039 (2008-03-11)
A simple and convenient synthesis of di(E)-arylidene-tetralone-spiro-glutarimides from Baylis-Hillman acetates via an interesting biscyclization strategy involving facile C-C and C-N bond formation is described. Also, one-pot multistep transformation of the Baylis-Hillman acetates into di(E)-arylidene-spiro-bisglutarimides is presented.
Pei-Qiang Huang et al.
Organic letters, 8(7), 1435-1438 (2006-03-28)
[reaction: see text] Using 5b as a common intermediate, the first asymmetric synthesis of (-)-epiquinamide (4) and a formal asymmetric synthesis of (-)-homopumiliotoxin 223G (2) is described. A key feature of our approach is the flexible introduction of a functionalized
Chuanjin Tian et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(45), 14305-14313 (2012-10-16)
The significance of the molecular chirality of drugs has been widely recognized due to the thalidomide tragedy. Most of the new drugs reaching the market today are single enantiomers, rather than racemic mixtures. However, many optically pure drugs, including thalidomide
Jianhua Ju et al.
Organic letters, 9(25), 5183-5186 (2007-11-14)
Lactimidomycin (LTM, 1) is a macrolide antitumor antibiotic with a glutarimide side chain from Streptomyces amphibiosporus ATCC53964. To further develop LTM and related analogues as drug candidates we have (i) improved LTM production by approximately 20 fold, (ii) identified three
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.