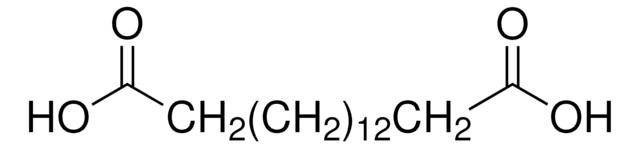Alle Fotos(2)
Wichtige Dokumente
177490
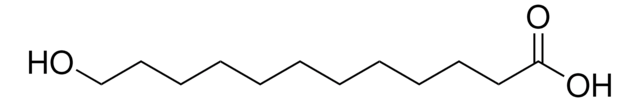
16-Hydroxyhexadecansäure
98%
Synonym(e):
16-Hydroxy-palmitinsäure, Junipersäure
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
HO(CH2)15CO2H
CAS-Nummer:
Molekulargewicht:
272.42
Beilstein:
1783998
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
solid
mp (Schmelzpunkt)
94-98 °C (lit.)
Funktionelle Gruppe
carboxylic acid
hydroxyl
SMILES String
OCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C16H32O3/c17-15-13-11-9-7-5-3-1-2-4-6-8-10-12-14-16(18)19/h17H,1-15H2,(H,18,19)
InChIKey
UGAGPNKCDRTDHP-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
16-Hydroxyhexadecanoic acid was used in the synthesis of dihydroxypalmitic acids. It was also used to induce the expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Sapa Hima Rani et al.
The Journal of biological chemistry, 285(49), 38337-38347 (2010-10-06)
A key step in the triacylglycerol (TAG) biosynthetic pathway is the final acylation of diacylglycerol (DAG) by DAG acyltransferase. In silico analysis has revealed that the DCR (defective in cuticular ridges) (At5g23940) gene has a typical HX(4)D acyltransferase motif at
Akihisa Abe et al.
Journal of biochemistry, molecular biology, and biophysics : JBMBB : the official journal of the Federation of Asian and Oceanian Biochemists and Molecular Biologists (FAOBMB), 6(1), 37-43 (2002-08-21)
We have found that omega-hydroxy palmitic acid (16-hydroxy palmitic acid, omega-HPA) has both cell growth inhibiting and cell death inducing actions on human lung adenosquamous carcinoma cell line H596 and adenocarcinoma cell line A549. Further, these effects were dose- and
Jong Ho Park et al.
Plant physiology and biochemistry : PPB, 46(11), 1015-1018 (2008-07-29)
Glycine-rich proteins (GRPs) belong to a large family of heterogenous proteins that are enriched in glycine residues. The expression of two GRP genes of Arabidopsis thaliana, AtGRP5 and AtGRP23, was induced by 16-hydroxypalmitic acid (HPA), a major component of cutin.
G D Rees et al.
Biochimica et biophysica acta, 1257(3), 239-248 (1995-08-03)
Five microbial lipases from Chromobacterium viscosum, Candida cylindracea, Pseudomonas (source Fluka), Pseudomonas (source Genzyme) and lipoprotein lipase ex Microbial (Genzyme) have been screened for lactonisation activity towards 16-hydroxyhexadecanoic acid (HHA) in a variety of different w/o microemulsion systems. With the
Jean-Paul Douliez
Journal of colloid and interface science, 271(2), 507-510 (2004-02-20)
The interaction between cutin and suberin monomers, i.e., omega -hydroxylpalmitic acid, alpha, omega -hexadecanedioic acid, alpha, omega --hexadecanediol, 12-hydroxylstearic acid, and phospholipid vesicles biomimicking the lipid structure of plant cell membranes has been studied by optical and transmission electron microscopy
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.