Alle Fotos(1)
Wichtige Dokumente
126462
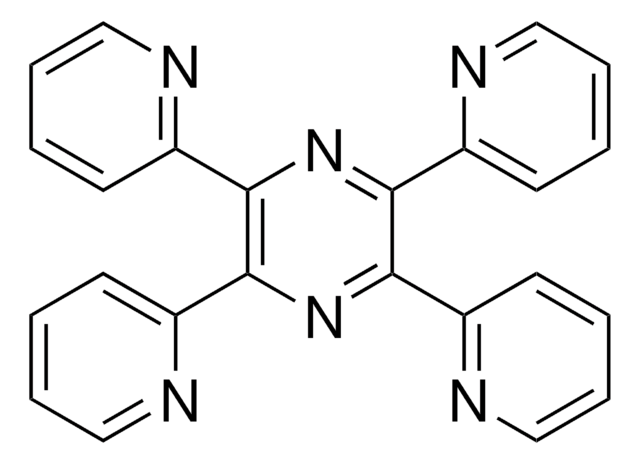
6,7-Dimethyl-2,3-di(2-pyridyl)chinoxalin
98%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C20H16N4
CAS-Nummer:
Molekulargewicht:
312.37
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
powder
mp (Schmelzpunkt)
191-193 °C (lit.)
SMILES String
Cc1cc2nc(-c3ccccn3)c(nc2cc1C)-c4ccccn4
InChI
1S/C20H16N4/c1-13-11-17-18(12-14(13)2)24-20(16-8-4-6-10-22-16)19(23-17)15-7-3-5-9-21-15/h3-12H,1-2H3
InChIKey
NACXMBPTPBZQHY-UHFFFAOYSA-N
Verwandte Kategorien
Anwendung
6,7-Dimethyl-2,3-di-(2-pyridyl)quinoxaline has been used as an internal standard to investigate the clinical pharmacokinetics of nelfinavir mesylate, a potent inhibitor of HIV-1 protease.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
E Y Wu et al.
Journal of chromatography. B, Biomedical sciences and applications, 695(2), 373-380 (1997-08-01)
Nelfinavir mesylate, a potent and orally bioavailable inhibitor of HIV-1 protease (Ki=2 nM), has undergone Phase III clinical evaluation in a large population of HIV-positive patients. A high-performance liquid chromatography analytical method was developed to determine the pharmacokinetic parameters of
B Louveau et al.
Biomedical chromatography : BMC, 30(12), 2009-2015 (2016-06-10)
A precise and accurate high-performance liquid chromatography (HPLC) quantification method of rifampicin in human plasma was developed and validated using ultraviolet detection after an automatized solid-phase extraction. The method was validated with respect to selectivity, extraction recovery, linearity, intra- and
Sara Baldelli et al.
Therapeutic drug monitoring, 36(6), 739-745 (2014-04-18)
Recently, the European Medicines Agency (EMA) has released new guidelines on the validation of bioanalytical methods. In this work, we compared the analytical performance of 2 high-performance liquid chromatography with tandem mass spectrometry methods designed for the quantification of the
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 126462-25G | |
| 126462-5G | 4061833320150 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.