Alle Fotos(2)
Wichtige Dokumente
12255
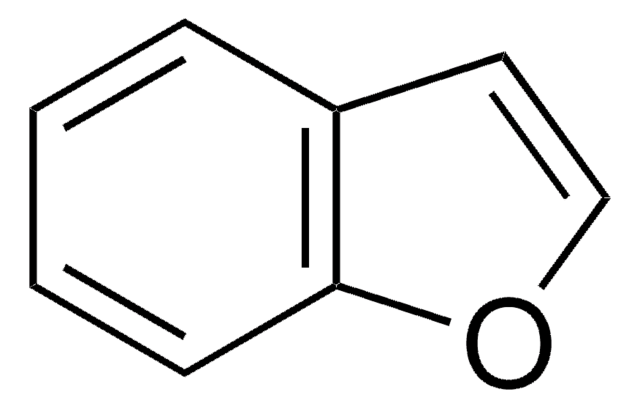
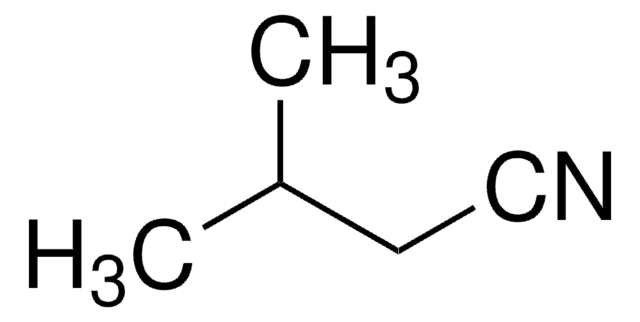
1,2-Benzisoxazol
≥95.0%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Empirische Formel (Hill-System):
C7H5NO
CAS-Nummer:
Molekulargewicht:
119.12
Beilstein:
2154
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥95.0%
Brechungsindex
n20/D 1.561 (lit.)
n20/D 1.563
bp
90-92 °C/15 mmHg (lit.)
Dichte
1.174 g/mL at 25 °C (lit.)
Lagertemp.
2-8°C
SMILES String
c1ccc2oncc2c1
InChI
1S/C7H5NO/c1-2-4-7-6(3-1)5-8-9-7/h1-5H
InChIKey
KTZQTRPPVKQPFO-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
1,2-Benzisoxazole derivative zonisamide is a novel antiepileptic drug and is effective for the treatment of partial seizures. 1,2-Benzisoxazole is a potential substrates of rabbit liver aldehyde oxidase.
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
186.8 °F - closed cup
Flammpunkt (°C)
86 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
D H Peters et al.
Drugs, 45(5), 760-787 (1993-05-01)
Zonisamide is a 1,2 benzisoxazole derivative and the first agent of this chemical class to be developed as an antiepileptic drug. It has shown activity in various animal models of epilepsy, and although a detailed mode of action awaits clarification
W Gristwood et al.
Xenobiotica; the fate of foreign compounds in biological systems, 18(8), 949-954 (1988-08-01)
1. Twelve oxygen and sulphur azaheterocycles were studied as potential substrates of rabbit liver aldehyde oxidase. Only benzoxazole and 1,2-benzisoxazole were found to be substrates. 2. Nine of the compounds inhibited the oxidation of quinazoline by aldehyde oxidase and in
Nan-Sook Hong et al.
Nature communications, 9(1), 3900-3900 (2018-09-27)
Developments in computational chemistry, bioinformatics, and laboratory evolution have facilitated the de novo design and catalytic optimization of enzymes. Besides creating useful catalysts, the generation and iterative improvement of designed enzymes can provide valuable insight into the interplay between the
Avneet Kaur et al.
Archiv der Pharmazie, 351(6), e1800008-e1800008 (2018-05-10)
A series of N-(2-(3,5-dimethoxyphenyl)benzoxazole-5-yl)benzamide derivatives (3am) was synthesized and evaluated for their in vitro inhibitory activity against COX-1 and COX-2. The compounds with considerable in vitro activity (IC50 < 1 μM) were evaluated in vivo for their anti-inflammatory potential by the carrageenan-induced
Min Bao et al.
International journal of biological macromolecules, 137, 537-544 (2019-06-25)
Studies on endo-inulinases from yeast are scarce, compared to those from other microbial sources. In this study, a novel endo-inulinase from Lipomyces starkeyi NRRL Y-11557 was identified, expressed in its soluble form, and characterized its physicochemically properties, together with its
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.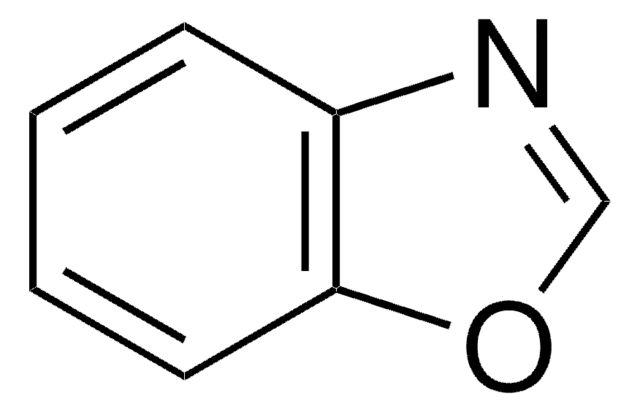
![Benzo[b]thiophen 98%](/deepweb/assets/sigmaaldrich/product/structures/207/595/6f99be5d-8490-46b4-aa55-51f75b9a9845/640/6f99be5d-8490-46b4-aa55-51f75b9a9845.png)