115754
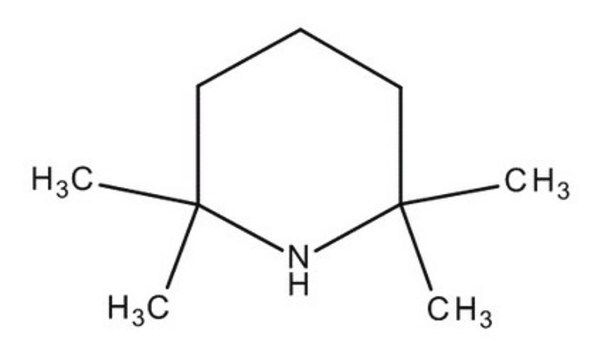
2,2,6,6-Tetramethylpiperidin
≥99%
Synonym(e):
2,2,6,6-tetramethylpiperidide, 2,2,6,6-tetramethylpiperidine, Norpempidine, TEMP, TMPH
About This Item
Empfohlene Produkte
Assay
≥99%
Brechungsindex
n20/D 1.445 (lit.)
bp
152 °C (lit.)
Dichte
0.837 g/mL at 25 °C (lit.)
SMILES String
CC1(C)CCCC(C)(C)N1
InChI
1S/C9H19N/c1-8(2)6-5-7-9(3,4)10-8/h10H,5-7H2,1-4H3
InChIKey
RKMGAJGJIURJSJ-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
- Allylated tertiary amines via allylic amination of allylic chlorides.
- Hydroxylamines via oxidation in the presence of oxone as an oxidant.
- Sulfenamide compounds by reacting with heterocyclic thiols in the presence of iodine as an oxidant.
- N-methylated amines via N-methylation by reacting with CO2 and phenylsilane.
- Propargylamines via three-component Mannich coupling reaction with aldehydes and alkynes.
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Met. Corr. 1 - Skin Corr. 1A - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
3 - Flammable liquids
WGK
WGK 2
Flammpunkt (°F)
98.6 °F - closed cup
Flammpunkt (°C)
37 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
COMU is a non-explosive coupling agent suitable for solution phase & solid phase peptide synthesis. Its activity meets or exceeds that of HATU and its water-soluble by-product are easily removed.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.