103543
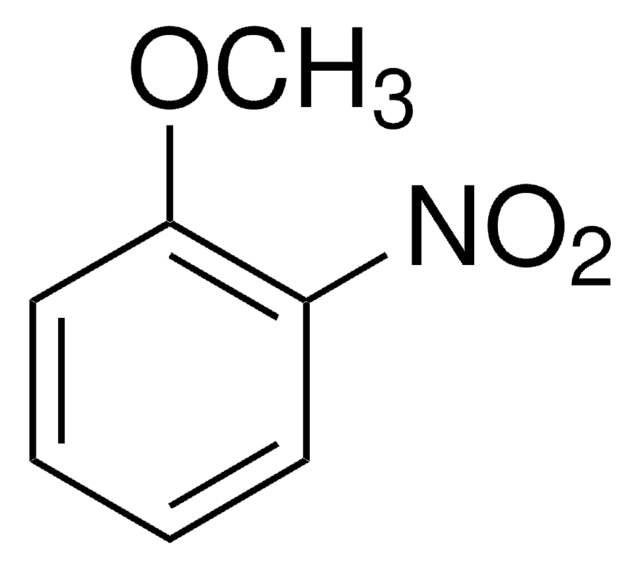
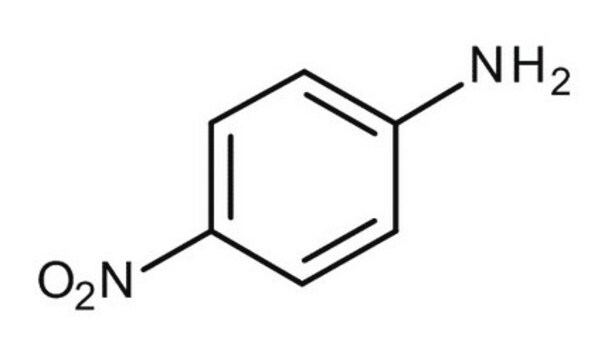
4-Nitroanisol
97%
Synonym(e):
1-Methoxy-4-nitro-benzol
About This Item
Empfohlene Produkte
Qualitätsniveau
Assay
97%
Form
solid
Dichte
1.233 g/mL at 25 °C (lit.)
Funktionelle Gruppe
nitro
SMILES String
COc1ccc(cc1)[N+]([O-])=O
InChI
1S/C7H7NO3/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3
InChIKey
BNUHAJGCKIQFGE-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
Biochem./physiol. Wirkung
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Aquatic Chronic 3 - Carc. 2
Lagerklassenschlüssel
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 1
Flammpunkt (°F)
266.0 °F - closed cup
Flammpunkt (°C)
130 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.