QBD10199
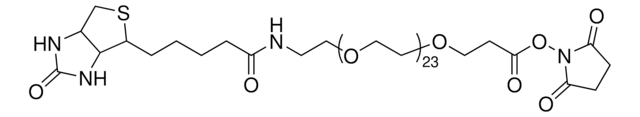
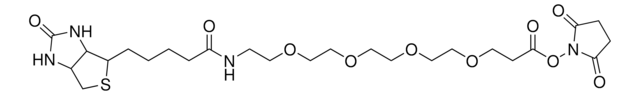
dPEG®4-biotin acid
>95% (HPLC)
Synonym(s):
Biotin-PEG-acid, Biotin-PEG4-COOH, Biotin-PEG4-acid, Carboxy-PEG4-biotin, PEG4-biotin acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C21H37N3O8S
Molecular Weight:
491.60
UNSPSC Code:
12352106
NACRES:
NA.22
Recommended Products
Assay
>95% (HPLC)
form
solid or viscous liquid
reaction suitability
reaction type: Biotinylations
reaction type: Pegylations
polymer architecture
shape: linear
functionality: monofunctional
shipped in
ambient
storage temp.
−20°C
Features and Benefits
dPEG4 biotin acid consists of biotin and a propionic acid-functionalized tetraethylene glycol derivative. Numerous functional or reactive groups can modify the terminal acid moiety, including the acylating agents N-hydroxysuccinimide (NHS) and 2,3,5,6-tetrafluorophenol (TFP). Also, dPEG4 biotin acid reacts directly with primary amines using a suitable carbodiimide such as 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC). The amphiphilic dPEG linker gives biotin excellent solubility in water and aqueous buffer, where biotin usually is poorly soluble. dPEG4 biotin acid offers significantly superior solubility and performance characteristics compared to the traditional, highly hydrophobic biotinylation reagent known as LC-biotin. Moreover, although dPEG4 biotin acid and LC-biotin have comparable linker lengths, dPEG4 biotin acid will not trigger the aggregation and precipitation of conjugated biomolecules, even at high levels of biotin incorporation on the biomolecule. In contrast, LC-biotin triggers the aggregation and precipitation of biomolecules with the incorporation of a few LC-biotin groups onto the biomolecule.
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Automate your Biotin tagging with Synple Automated Synthesis Platform (SYNPLE-SC002)
Legal Information
Products Protected under U.S. Patent #s 7,888,536 & 8,637,711 and European Patent #s 1,594,440 & 2,750,681
dPEG is a registered trademark of Quanta BioDesign
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Alexander Kuzmin et al.
Bioconjugate chemistry, 21(11), 2076-2085 (2010-10-23)
The utility of catalyst-free azide-alkyne [3 + 2] cycloaddition for the immobilization of a variety of molecules onto a solid surface and microbeads was demonstrated. In this process, the surfaces are derivatized with aza-dibenzocyclooctyne (ADIBO) for the immobilization of azide-tagged
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
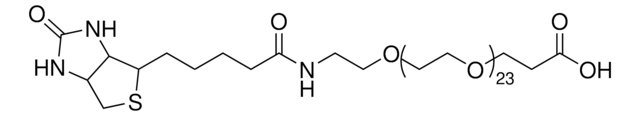
![O-[2-(Biotinyl-amino)ethyl]-O′-(2-carboxyethyl)polyethylene glycol Mp 3,000](/deepweb/assets/sigmaaldrich/product/structures/285/242/a1e6e88b-5b7d-43b5-9bb0-18dcbfdccf43/640/a1e6e88b-5b7d-43b5-9bb0-18dcbfdccf43.png)