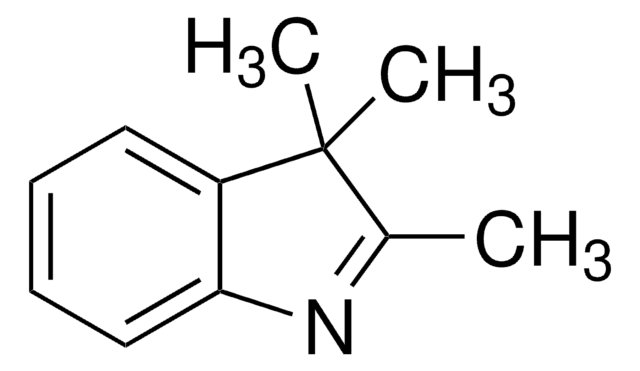
304484
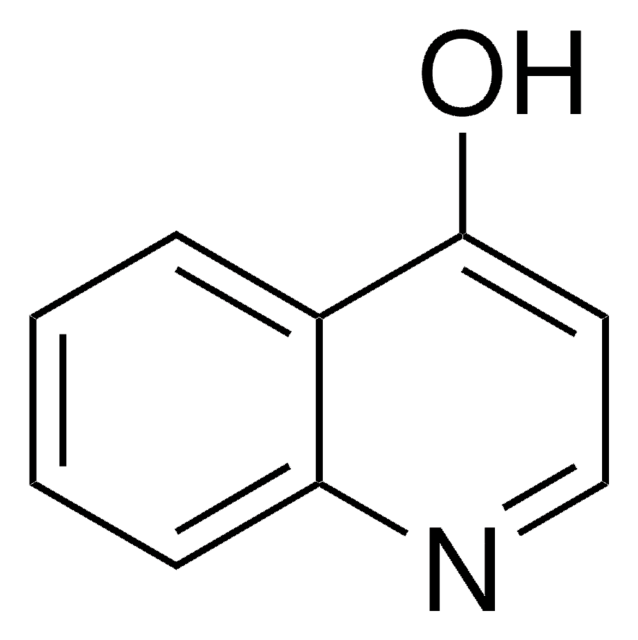
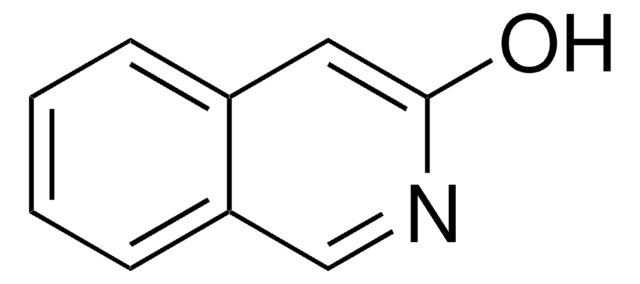
6-Hydroxyquinoline
95%
Synonym(s):
6-Quinolinol, 6-Hydroxyquinoline
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C9H7NO
CAS Number:
Molecular Weight:
145.16
Beilstein:
113196
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
188-190 °C (lit.)
SMILES string
Oc1ccc2ncccc2c1
InChI
1S/C9H7NO/c11-8-3-4-9-7(6-8)2-1-5-10-9/h1-6,11H
InChI key
OVYWMEWYEJLIER-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
6-Hydroxyquinoline is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. The excited-state proton transfer and geminate recombination of 6-hydroxyquinoline encaged in catalytic Na+-exchanged faujasite zeolites X and Y have been explored by measuring steady-state and picosecond time-resolved spectra.
Application
6-Hydroxyquinoline was used in synthesis of 2,6-substituted-benzo[d]thiazole analogs and 2,4-substituted-benzo[d]thiazole analogs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Katharigatta Narayanaswamy Venugopala et al.
European journal of medicinal chemistry, 65, 295-303 (2013-06-04)
A novel and efficient one pot synthesis was developed for 2,6-substituted-benzo[d]thiazole analogues 4a-k and 2,4-substituted-benzo[d]thiazole analogues 4l-pvia three component condensation reaction of substituted arylaldehyde, 2-amino-6-halo/4-methyl-benzo[d]thiazole and 2-naphthol or 6-hydroxyquinoline in presence of 10% w/v NaCl in water by microwave method.
Yu-Hui Liu et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 128, 280-284 (2014-04-01)
6-Hydroxyquinoline (6HQ) is an ideal photoacid system for exploring excited-state proton transfer (ESPT) reactions. We have previously (Mahata et al. (2002)) shown that the ESPT reaction between 6HQ and trimethylamine (TMA) leads to an "unusual" emission in the 440-450 nm
Mohan Singh Mehata
The journal of physical chemistry. B, 112(28), 8383-8386 (2008-06-20)
A hydrogen-bonded network formed between 6-hydroxyquinoline (6-HQ) and acetic acid (AcOH) has been characterized using a time-resolved fluorescence technique. In the bridged hydrogen-bonded complex of cis-6-HQ and AcOH, an excited-state reaction proceeds via proton transfer along the hydrogen bond, resulting
Yu-Hui Liu et al.
The journal of physical chemistry. A, 115(1), 19-24 (2010-12-15)
Spectroscopic studies on excited-state proton transfer (ESPT) of hydroxyquinoline (6HQ) have been performed in a previous paper. And a hydrogen-bonded network formed between 6HQ and acetic acid (AcOH) in nonpolar solvents has been characterized. In this work, a time-dependent density
Junhong Qian et al.
Physical chemistry chemical physics : PCCP, 12(39), 12562-12569 (2010-08-21)
Photophysical properties of the organocatalyst cupreidine (CPD) and its chromophoric building block 6-hydroxyquinoline (6HQ) in protic and nonprotic polar solvents (methanol and acetonitrile) were investigated by means of UV-vis absorption, and steady state and time resolved fluorescence spectroscopy. The effects
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service