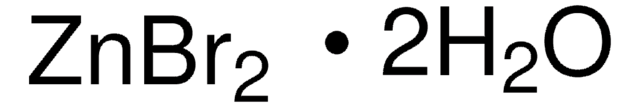223883
Zinc iodide
≥98%
Synonym(s):
Diiodozinc, Zinc diiodide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ZnI2
CAS Number:
Molecular Weight:
319.20
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.23
grade:
for analytical purposes
form:
powder
Recommended Products
grade
for analytical purposes
Assay
≥98%
form
powder
reaction suitability
reagent type: catalyst
core: zinc
mp
445 °C (lit.)
density
4.74 g/mL at 25 °C (lit.)
SMILES string
I[Zn]I
InChI
1S/2HI.Zn/h2*1H;/q;;+2/p-2
InChI key
UAYWVJHJZHQCIE-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Zinc iodide can be used:
- As an activator to synthesize poly(vinyl methyl ether) via living cationic polymerization. ZnI2 is known to produce living vinyl ether polymerizations.
- To prepare precursor solutions for lead halide perovskite solar cells. The addition of ZnI2 leads to higher stability against environmental moisture when introduced into perovskite cells.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2 - STOT RE 2 Oral
Target Organs
Thyroid
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Loreta A Muscarella et al.
ACS applied materials & interfaces, 11(19), 17555-17562 (2019-04-17)
We present a one-step method to produce air-stable, large-grain mixed cationic lead perovskite films and powders under ambient conditions. The introduction of 2.5 % of Zn(II), confirmed by X-ray diffraction (XRD), results in stable thin films which show the same
N Mayer-Gostan et al.
Cell and tissue research, 289(1), 53-61 (1997-07-01)
The saccular membranes of trout (Oncorhynchus mykiss) and turbot (Scophthalmus maximus) were examined to characterize specialized epithelial cells that might be responsible for ion exchange. The approach for localizing cell types was new for this tissue, as observations were made
Lei Han et al.
Inorganic chemistry, 46(5), 1511-1513 (2007-02-08)
Hydrothermal reaction of 4,4-trimethylenedipyridine (tmdp) with ZnI2 under 175 degrees C yields a novel compound, {[Zn2I4(tmdp)2]n.[Zn2I4(tmdp)2]n}, which has a chiral infinite double-stranded helical structure consisting of two single-stranded helices of the same handedness.
Jie Ma et al.
Chemistry, an Asian journal, 5(10), 2214-2220 (2010-08-03)
Transition-metal-activated alkynes or allenes can accept nucleophilic attack and undergo direct addition of the nucleophiles to the unsaturated bonds or trigger subsequent rearrangement reactions. This chemistry has witnessed increasing development in recent years. In this report, we have focused on
C Slomianny et al.
The Journal of protozoology, 37(6), 465-470 (1990-11-01)
We have used ultrastructural techniques in different malarial species to demonstrate a lysosomal system. First, we have tried to localize acid phosphatase, a typical lysosomal label. Its activity was localized in the endoplasmic reticulum and in endocytic vesicles, and in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service