1361009
USP
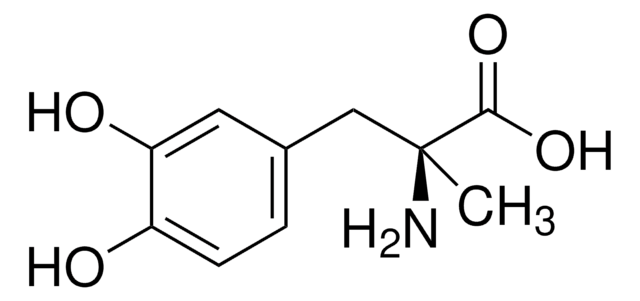
Levodopa
United States Pharmacopeia (USP) Reference Standard
Sinonimo/i:
3,4-Dihydroxy-L-phenylalanine, 3-(3,4-Dihydroxyphenyl)-L-alanine, L-3-Hydroxytyrosine, L-DOPA, Levodopa
About This Item
Prodotti consigliati
Grado
pharmaceutical primary standard
Famiglia di API
levodopa
Produttore/marchio commerciale
USP
Punto di fusione
276-278 °C (lit.)
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
N[C@@H](Cc1ccc(O)c(O)c1)C(O)=O
InChI
1S/C9H11NO4/c10-6(9(13)14)3-5-1-2-7(11)8(12)4-5/h1-2,4,6,11-12H,3,10H2,(H,13,14)/t6-/m0/s1
WTDRDQBEARUVNC-LURJTMIESA-N
Informazioni sul gene
human ... DRD3(1814)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- Carbidopa and Levodopa Tablets
- Carbidopa and Levodopa Orally Disintegrating Tablets
- Carbidopa and Levodopa Extended-Release Tablets
Risultati analitici
Altre note
Prodotti correlati
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documenti section.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.