U211150
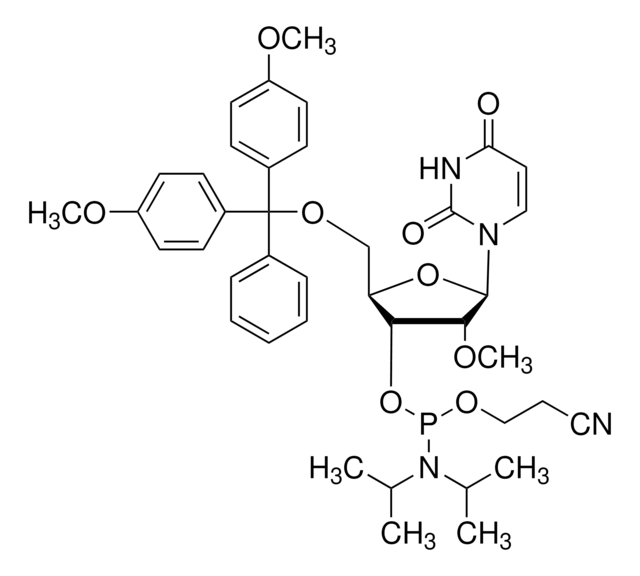
DMT-2′O-Methyl-rU Phosphoramidite
configured for ÄKTA® and OligoPilot®
Sinonimo/i:
5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-O-methyl-uridine, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite], DMT-2′O-Methyl-rU amidite
About This Item
Prodotti consigliati
Origine biologica
non-animal source (no BSE/TSE risk)
Livello qualitativo
Nome Commerciale
Proligo Reagents
Saggio
≥99% (31P-NMR)
≥99.0% (reversed phase HPLC)
Stato
powder
tecniche
oligo synthesis: suitable
Impurezze
≤0.1% single unspecified Impurity (reversed phase HPLC)
≤0.3% mU2 (reversed phase HPLC, Hydrolysate)
≤0.3% mU3 (reversed phase HPLC, DMT-rme)
≤0.3% water content (Karl Fischer)
≤0.5% P(III) Impurities 100-169ppm (31P-NMR)
≤1.0% mU1 (reversed phase HPLC, DMT-rUme-DMT)
≤3% residual Solvent content
Colore
white to off-white
λ
conforms (UV/VIS Identity)
Compatibilità
conforms to structure for H-NMR
conforms to structure for LC-MS
configured for ÄKTA® and OligoPilot®
Profilo del nucleoside
base: uridine
base protecting group: none
2' protecting group: methyl
5' protecting group: DMT
deprotection: fast/standard
Temperatura di conservazione
2-8°C
Stringa SMILE
CO[C@@H]1[C@H](OP(OCCC#N)N(C(C)C)C(C)C)[C@@H](COC(c2ccccc2)(c3ccc(OC)cc3)c4ccc(OC)cc4)O[C@H]1N5C=CC(=O)NC5=O
InChI
1S/C40H49N4O9P/c1-27(2)44(28(3)4)54(51-25-11-23-41)53-36-34(52-38(37(36)49-7)43-24-22-35(45)42-39(43)46)26-50-40(29-12-9-8-10-13-29,30-14-18-32(47-5)19-15-30)31-16-20-33(48-6)21-17-31/h8-10,12-22,24,27-28,34,36-38H,11,25-26H2,1-7H3,(H,42,45,46)/t34-,36-,37-,38-,54?/m1/s1
UVUOJOLPNDCIHL-XKZJCBTISA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Caratteristiche e vantaggi
- High yield of crude oligonucleotides
- Compatible with DNA synthesis
- Can be employed together with DNA or RNA phosphoramidites in the same synthesis to produce mixmer oligonucleotides
- Recommended deprotection conditions are 8 hours at 55 °C using concentrated ammonia solution, or with AMA (concentrated ammonia/ 40% aqueous methylamine I/I, v/v) for 10 minutes at 65 °C
- Purification and other downstream processing of fully modified 2′OMethyl RNA oligonucleotides are simpler than in the case of RNA, as no special precautions are required to provide protection against nucleolytic degradation
- Synthesis of 2′O-Methyl RNA oligonucleotides is similar to standard DNA synthesis but requires an elongated coupling time (recommended is 6 minutes compared to 90 seconds for DNA monomers)
- 2′O-Methyl RNA phosphoramidites are also available with fast deprotection chemistry
Altre note
- Diagnostic probes
- Aptamer and ribozyme development
- Mixed 2′O-Methyl-RNA/DNA antisense molecules
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.