T6575
TOFA
≥98% (HPLC)
Sinonimo/i:
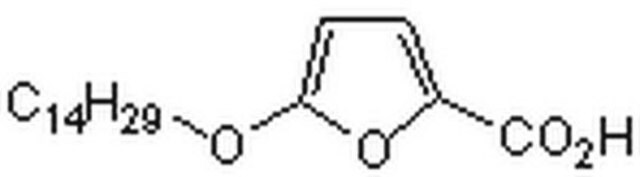
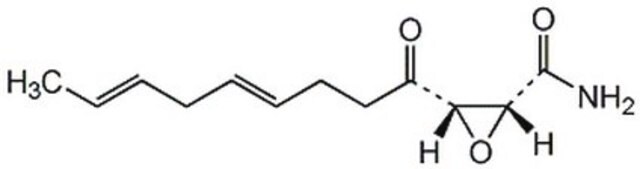
5-(Tetradecyloxy)-2-furoic acid
About This Item
Prodotti consigliati
Saggio
≥98% (HPLC)
Stato
powder
Colore
white to beige
Punto di fusione
112-115 °C
Solubilità
DMSO: 2 mg/mL, clear
Temperatura di conservazione
−20°C
Stringa SMILE
CCCCCCCCCCCCCCOc1ccc(o1)C(O)=O
InChI
1S/C19H32O4/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-22-18-15-14-17(23-18)19(20)21/h14-15H,2-13,16H2,1H3,(H,20,21)
CZRCFAOMWRAFIC-UHFFFAOYSA-N
Applicazioni
Azioni biochim/fisiol
Nota sulla preparazione
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.