SAB4200800
Anti-Collagen, Type X antibody, Mouse monoclonal
clone COL-10, purified from hybridoma cell culture
Sinonimo/i:
Anti-COL10A1
About This Item
Prodotti consigliati
Origine biologica
mouse
Forma dell’anticorpo
purified from hybridoma cell culture
Tipo di anticorpo
primary antibodies
Clone
COL-10, monoclonal
Stato
buffered aqueous solution
PM
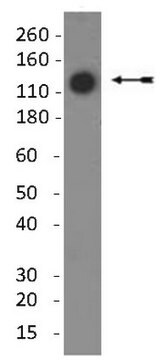
~60 kDa
Reattività contro le specie
deer, porcine, human
Confezionamento
antibody small pack of 25 μL
Concentrazione
~1 mg/mL
tecniche
immunoblotting: suitable
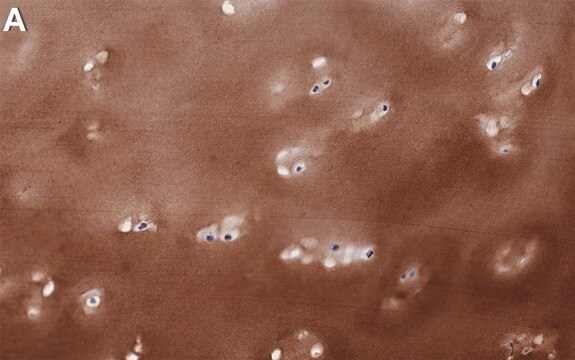
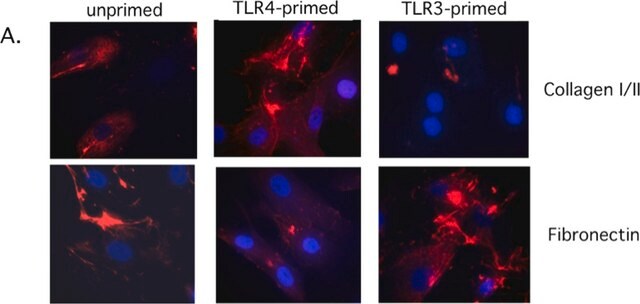
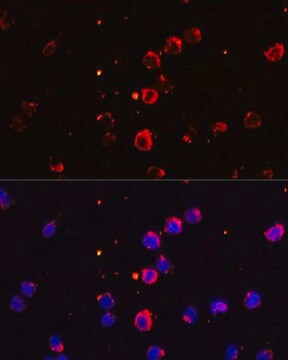
immunofluorescence: 5-10 μg/mL using human osteosarcoma SaOS-2 cells
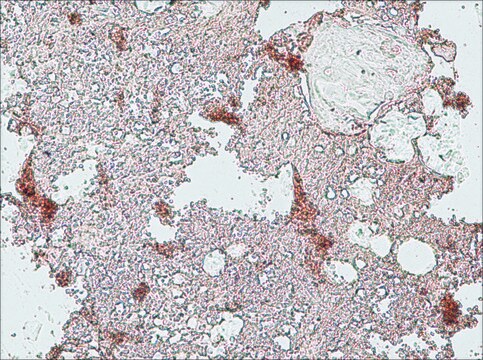
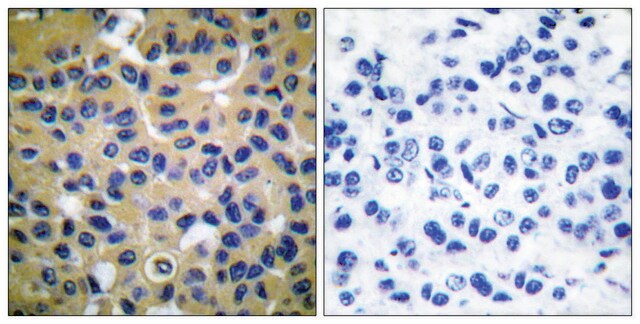
immunohistochemistry: suitable
Isotipo
IgM
N° accesso UniProt
Condizioni di spedizione
dry ice
Temperatura di conservazione
−20°C
modifica post-traduzionali bersaglio
unmodified
Informazioni sul gene
human ... COL10A1(1300)
Categorie correlate
Descrizione generale
Type X collagen,also known as Collagen alpha-1(X) chain (COL10A1), is a product of hypertrophic chondrocytes. It shares a similar domain structure with type VIII collagen. In addition, both collagen types represent major components of hexagonal lattice structure, in which the collagen molecules link together by interactions involving the non-triple-helical end regions. Despite these similarities, a distinct tissue distribution has been found for these two molecules: type VIII collagen is distributed in various tissues, whereas type X is restricted to normal fetal hypertrophic cartilage in the growth zones of long bones, vertebrae and ribs and in adult (> 21 yr) thyroid cartilage. It is also found in bone fracture callus, osteoarthritic cartilage and chondrogenic neoplasms, and may be involved in cartilage mineralization. Type X collagen is non-fibrillar, but forms fine pericellular filaments in association with cartilage collagen. It interacts with matrix proteins, such as connexin V, chondrocalcein, collagen II and proteoglycans, as well as with Ca2+ . Mutations in this gene are associated with schmid metaphyseal chondroplasia (MCDS).
The development of antibodies against collagens has provided a powerful method for examining the distribution of these connective tissue proteins and for investigation of epithelial-mesenchymal interactions, tumorigenesis and basement membrane biology in ontogeny and epithelial differentiation.8 Antibodies that react specifically with collagen type X are useful for the study of specific differential tissue expression and the localization of collagen type X.
Immunogeno
Applicazioni
Stato fisico
Altre note
Non trovi il prodotto giusto?
Prova il nostro Motore di ricerca dei prodotti.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Non trovi la versione di tuo interesse?
Se hai bisogno di una versione specifica, puoi cercare il certificato tramite il numero di lotto.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.