H5529
Heregulin-α, EGF Domain human
recombinant, expressed in E. coli, lyophilized powder, suitable for cell culture, ≥97% (SDS-PAGE)
Sinonimo/i:
HRG-α
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Origine biologica
human
Livello qualitativo
Ricombinante
expressed in E. coli
Saggio
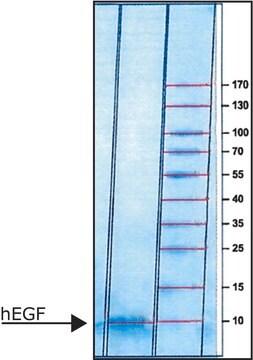
≥97% (SDS-PAGE)
Stato
lyophilized powder
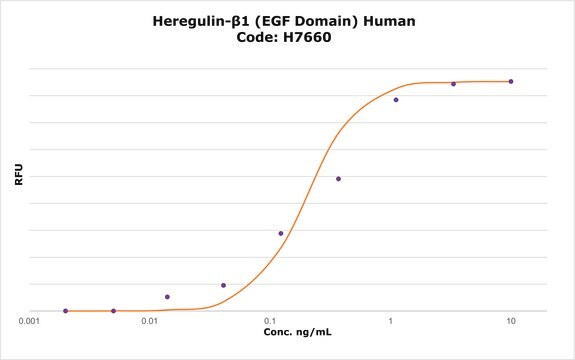
Potenza
10-50 ng/mL
PM
protein 8 kDa (reducing conditions)
protein ~7 kDa (predicted)
Confezionamento
pkg of 50 μg
tecniche
cell culture | mammalian: suitable
Impurezze
endotoxin, tested
N° accesso UniProt
Temperatura di conservazione
−20°C
Informazioni sul gene
human ... NRG1(3084)
Azioni biochim/fisiol
Heregulin (HRG) is the human homologue to the neu differentiation factor (NDF) in rat. The heregulin family members contain one EGF-like motif and an IgD-like motif in the extracellular domain. They bind to ErbB-2, ErbB-3, and ErbB-4 (receptors closely related to EGFR). HRG-α and HRG-β isoforms differ slightly in the EGF domain due to alternate splicing. HRG-β isoforms are further subdivided into β1, β2, and β3 isoforms, which show identical binding and activation characteristics. Both α and β HRG isoforms bind to ErbB-3 and ErbB-4 homodimers, but not directly to ErbB-2. HRG-α binding to ErbB-3 and ErbB-4 is reported to be approximately 100-fold weaker than that of HRG-β. When ErbB-2 is combined into a heterodimer with ErbB-3 or ErbB-4, the binding affinities of both α and β isoforms are substantially improved. HRGs are mitogenic for Schwann cells in culture and weakly to moderately mitogenic for a variety of epithelial cells, including mammary, ovarian, lung, and gastric cells. HRGs inhibit proliferation and induce differentation in some tumor cell lines, such as certain mammary tumor cells, which are arrested at the G2M phase. HRGs also induce expression of acetylcholine receptors and possibly other molecules in muscle cells at newly formed neuromuscular synapses, suggesting they may play a role in neuromuscular synapse maturation and maintenance.
Stato fisico
Lyophilized from a 0.2 μm filtered solution in phosphate buffered saline, pH 7.4, containing 50 μg bovine serum albumin per 1 μg as a carrier protein.
Risultati analitici
The bioactivity is tested in culture using a proliferation assay with the human cell line MCF-7.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
J T Jones et al.
FEBS letters, 447(2-3), 227-231 (1999-04-24)
ErbB receptor activation is a complex process and is dependent upon the type and number of receptors expressed on a given cell. Previous studies with defined combinations of ErbB receptors expressed in mammalian cells have helped elucidate specific biological responses
Z Aguilar et al.
Oncogene, 18(44), 6050-6062 (1999-11-11)
The heregulins are a family of ligands with ability to induce phosphorylation of the p185HER-2/neu receptor. Various investigators have reported a variety of responses of mouse and human breast and ovarian cells to this family of ligands including growth stimulation
S Y Baek et al.
Developmental neuroscience, 20(6), 512-517 (1998-12-22)
Neu differentiation factor (NDF), a 44-kD polypeptide, is a member of the neuregulin family which also includes glial growth factor, heregulin and acetylcholine-receptor-inducing activity. Previous studies have demonstrated that NDF/glial growth factor/heregulin/acetylcholine-receptor-including activity are products of neurons and mediate proliferation
Neu differentiation factor (NDF) and the neuregulin (NRG) family.
Yarden, Y., Wen, D. et al.
The Cytokine Handbook, 146-146 (1998)
Take your partners, please--signal diversification by the erbB family of receptor tyrosine kinases.
R J Daly
Growth factors (Chur, Switzerland), 16(4), 255-263 (1999-07-31)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.