G115080
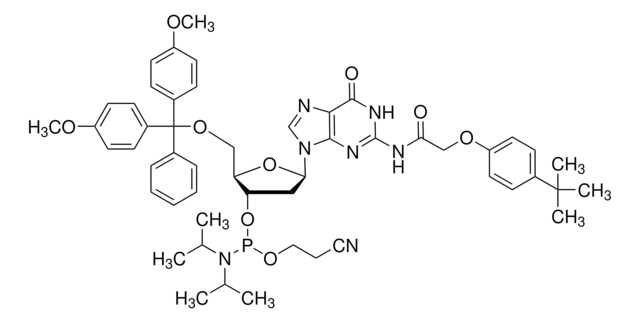
DMT-dG(dmf) Phosphoramidite
configured for PerkinElmer, configured for Polygen
Sinonimo/i:
DMT-dG(dmf) Amidite, N-[(dimethylamino)methylene]-5′-O-[bis(4-methoxyphenyl)phenylmethyl]-2′-deoxyguanosine, 3′-[2-cyanoethyl N,N-bis(1-methylethyl)phosphoramidite], N2-Dimethylformamidine-5′-O-(4,4′-dimethoxytrityl)-2′-deoxyguanosine-3′-O-[O-(2-cyanoethyl)-N,N′-diisopropylphosphoramidite]
About This Item
Prodotti consigliati
Tipo
for DNA synthesis
Nome Commerciale
Proligo Reagents
Saggio
≥99% (31P-NMR)
≥99.0% (reversed phase HPLC)
Forma fisica
powder
tecniche
oligo synthesis: suitable
Colore
white to off-white
λ
conforms (UV/VIS Identity)
Compatibilità
configured for PerkinElmer
configured for Polygen
Profilo del nucleoside
base: deoxyguanosine
base protecting group: DMF
2' protecting group: none
5' protecting group: DMT
deprotection: fast/standard
Temperatura di conservazione
-10 to -25°C
Stringa SMILE
COc1ccc(cc1)C(OC[C@H]2O[C@H](C[C@@H]2OP(OCCC#N)N(C(C)C)C(C)C)n3cnc4C(=O)NC(\N=C/N(C)C)=Nc34)(c5ccccc5)c6ccc(OC)cc6
InChI
1S/C43H53N8O7P/c1-29(2)51(30(3)4)59(56-24-12-23-44)58-36-25-38(50-28-45-39-40(50)47-42(48-41(39)52)46-27-49(5)6)57-37(36)26-55-43(31-13-10-9-11-14-31,32-15-19-34(53-7)20-16-32)33-17-21-35(54-8)22-18-33/h9-11,13-22,27-30,36-38H,12,24-26H2,1-8H3,(H,47,48,52)/b46-27-/t36-,37+,38+,59?/m0/s1
YRQAXTCBMPFGAN-UJASEYITSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
- dG(dmf) is deprotected faster than the conventional dG(ib): thedeprotection time in concentrated ammonia is reduced to 2 hours at55 °C or 1 hour at 65 °C
- The dG(dmf)-monomer is especially suitable for G-rich sequences:incomplete deprotection is greatly reduced in comparison with theconventional dG(ib)-monomer
- dG(dmf)-phosphoramidite is as stable in solution as the standard dA(bz)-,dC(bz)- and dT-phosphoramidites
- dG(dmf)-phosphoramidite can directly substitute for dG(ib)-phosphoramidite
- No change is required in the reagents commonly used for DNA synthesis(except a low concentration iodine oxidizer i.e., 0.02 M in iodine, should beemployed)DMT-dG(dmf) Phosphoramidite is configured for Expedite™ and PolyGen® Synthesizers.
Note legali
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.