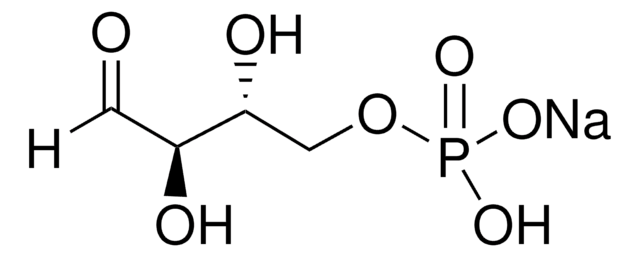
F3627
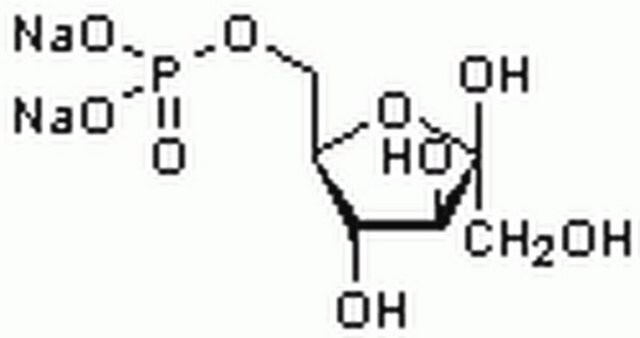
D-Fructose 6-phosphate disodium salt hydrate
≥98%, amorphous powder
Sinonimo/i:
Sodium (2R,3R,4S)-2,3,4,6-tetrahydroxy-5-oxohexyl phosphate
About This Item
Prodotti consigliati
Origine biologica
bacterial (Corynebacterium)
Livello qualitativo
Saggio
≥98%
Forma fisica
amorphous powder
Impurezze
<0.05 mol % fructose 1,6-diphosphate
<1.5 mol % glucose 6-phosphate
Colore
white to off-white
Solubilità
H2O: 100 mg/mL, clear to slightly hazy, colorless to faintly yellow
Cationi in tracce
Na: 14.6-15.6% (dry basis)
applicazioni
agriculture
Temperatura di conservazione
−20°C
Stringa SMILE
O.[Na+].[Na+].OC[C@@]1(O)O[C@H](COP([O-])([O-])=O)[C@@H](O)[C@@H]1O
InChI
1S/C6H13O9P.2Na.H2O/c7-2-6(10)5(9)4(8)3(15-6)1-14-16(11,12)13;;;/h3-5,7-10H,1-2H2,(H2,11,12,13);;;1H2/q;2*+1;/p-2/t3-,4-,5+,6-;;;/m1.../s1
VSCMQICEHMPOEC-HTKRKRNRSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
Azioni biochim/fisiol
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Review the 10 steps of glycolysis in the Embden-Meyerhof-Parnas glycolytic pathway. Easily compare reaction stages and buy the enzymes for your life science research.
Neoplastic cells are highly dependent on the de novo synthesis of nucleotides to maintain sufficient pools to support DNA replication and the production of RNA.
We presents an article about the Warburg effect, and how it is the enhanced conversion of glucose to lactate observed in tumor cells, even in the presence of normal levels of oxygen. Otto Heinrich Warburg demonstrated in 1924 that cancer cells show an increased dependence on glycolysis to meet their energy needs, regardless of whether they were well-oxygenated or not.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.