B8416
Bleomycin sulfate from Streptomyces verticillus
crystalline, suitable for cell culture, BioReagent
Sinonimo/i:
Blenoxane, Bleo, Blexane
About This Item
Prodotti consigliati
Nome del prodotto
Bleomycin sulfate from Streptomyces verticillus, 1.5-2.0 units/mg solid, BioReagent, suitable for cell culture
Nome Commerciale
BioReagent
Livello qualitativo
Stato
powder or crystals
Attività specifica
1.5-2.0 units/mg solid
tecniche
cell culture | mammalian: suitable
Colore
white to off-white
Solubilità
H2O: 20 mg/mL, clear, colorless
Spettro attività antibiotica
fungi
Modalità d’azione
DNA synthesis | interferes
Temperatura di conservazione
2-8°C
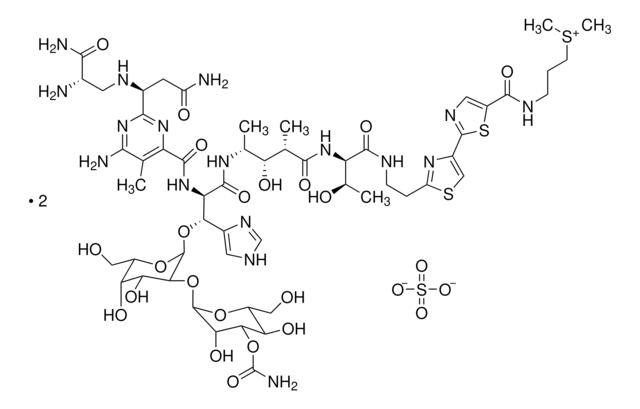
Stringa SMILE
[O-]S([O-])(=O)=O.C[C@@H](O)[C@@H](NC(=O)[C@@H](C)[C@H](O)[C@@H](C)NC(=O)[C@H](NC(=O)c1nc(nc(N)c1C)[C@H](CC(N)=O)NC[C@H](N)C(N)=O)[C@@H](O[C@@H]2O[C@@H](CO)[C@@H](O)[C@H](O)[C@@H]2O[C@@H]3O[C@H](CO)[C@@H](O)[C@@H](OC(N)=O)[C@@H]3O)c4c[nH]cn4)C(=O)NCCc5nc(cs5)-c6ncc(s6)C(=O)NCCC[S+](C)C.C[C@@H](O)[C@@H](NC(=O)[C@@H](C)[C@H](O)[C@@H](C)NC(=O)[C@H](NC(=O)c7nc(nc(N)c7C)[C@H](CC(N)=O)NC[C@H](N)C(N)=O)[C@@H](O[C@@H]8O[C@@H](CO)[C@@H](O)[C@H](O)[C@@H]8O[C@@H]9O[C@H](CO)[C@@H](O)[C@@H](OC(N)=O)[C@@H]9O)c%10c[nH]cn%10)C(=O)NCCc%11nc(cs%11)-c%12ncc(s%12)C(=O)NCCC[S+](C)C
InChI
1S/2C55H83N17O21S3.H2O4S/c2*1-20-33(69-46(72-44(20)58)25(12-31(57)76)64-13-24(56)45(59)82)50(86)71-35(41(26-14-61-19-66-26)91-54-43(39(80)37(78)28(16-73)90-54)92-53-40(81)42(93-55(60)88)38(79)29(17-74)89-53)51(87)67-22(3)36(77)21(2)47(83)70-34(23(4)75)49(85)63-10-8-32-68-27(18-94-32)52-65-15-30(95-52)48(84)62-9-7-11-96(5)6;1-5(2,3)4/h2*14-15,18-19,21-25,28-29,34-43,53-54,64,73-75,77-81H,7-13,16-17,56H2,1-6H3,(H13-,57,58,59,60,61,62,63,66,67,69,70,71,72,76,82,83,84,85,86,87,88);(H2,1,2,3,4)/t2*21-,22+,23+,24-,25-,28-,29+,34+,35+,36-,37+,38+,39-,40-,41-,42+,43-,53-,54-;/m00./s1
OOXTWFJZZAJGKA-CNLAFNBISA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
Azioni biochim/fisiol
Componenti
Avvertenza
Nota sulla preparazione
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Carc. 2 - Muta. 1B - Repr. 2
Codice della classe di stoccaggio
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
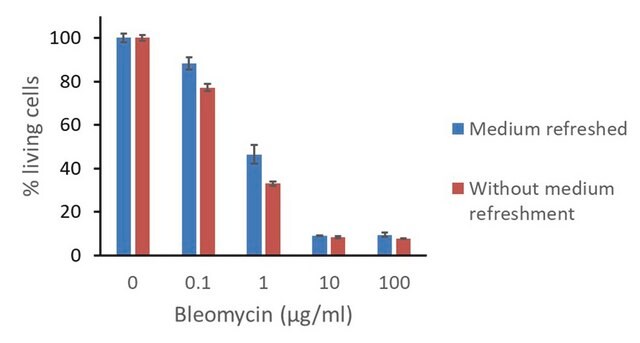
Antibiotic kill curve is a dose response experiment in which mammalian cells are subjected to increasing amounts of selection antibiotic
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.