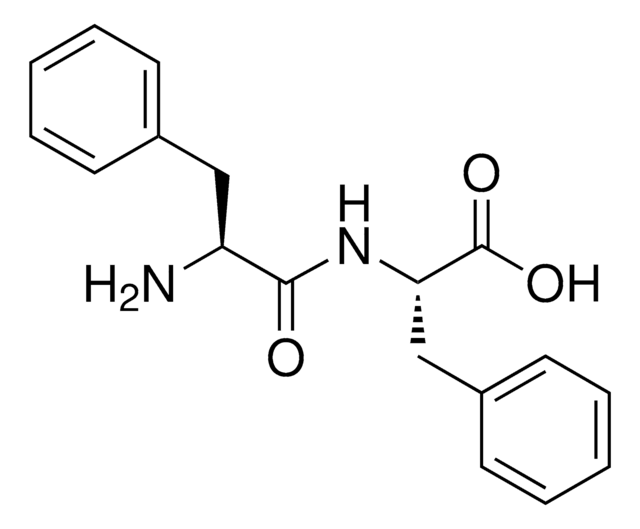
A3128
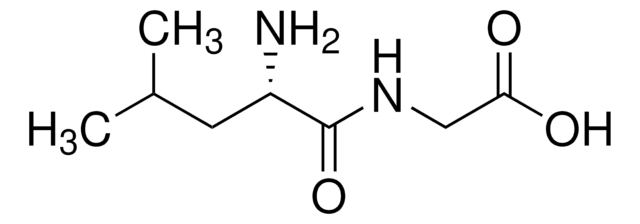
Ala-Phe
≥98% (TLC)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
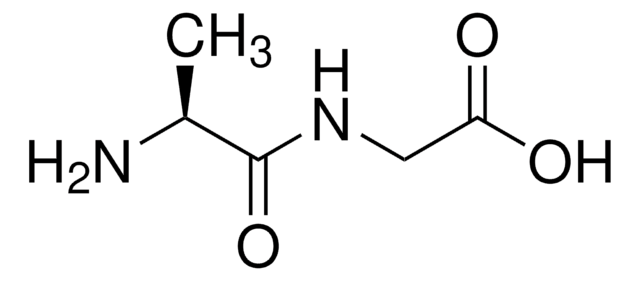
C12H16N2O3
Numero CAS:
Peso molecolare:
236.27
Numero MDL:
Codice UNSPSC:
12352202
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Nome del prodotto
Ala-Phe,
Saggio
≥98% (TLC)
Livello qualitativo
Stato
powder
Colore
white to off-white
Temperatura di conservazione
−20°C
Stringa SMILE
C[C@H](N)C(=O)N[C@@H](Cc1ccccc1)C(O)=O
InChI
1S/C12H16N2O3/c1-8(13)11(15)14-10(12(16)17)7-9-5-3-2-4-6-9/h2-6,8,10H,7,13H2,1H3,(H,14,15)(H,16,17)/t8-,10-/m0/s1
OMNVYXHOSHNURL-WPRPVWTQSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Amino Acid Sequence
Ala-Phe
Azioni biochim/fisiol
Alanyl dipeptides such as ala-leu, ala-lys, ala-gly, ala-pro, ala-tyr and ala-phe may be used in physicochemical studies or to evaluate dipeptide separation technologies. Alanyl dipeptides may also be used for studying cell uptake mechanisms, dipeptide metabolism or cell growth supplementation benefits.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Yasuhito Shomura et al.
Protein science : a publication of the Protein Society, 21(5), 707-716 (2012-03-13)
BacD is an ATP-dependent dipeptide ligase responsible for the biosynthesis of L-alanyl-L-anticapsin, a precursor of an antibiotic produced by Bacillus spp. In contrast to the well-studied and phylogenetically related D-alanine: D-alanine ligase (Ddl), BacD synthesizes dipeptides using L-amino acids as
Elizabeth A Girnys et al.
Chemical biology & drug design, 75(1), 35-39 (2009-12-04)
Myocardial ischemia and other acute coronary syndromes are leading causes of death worldwide, and often result from a thrombus that blocks an atherosclerotic coronary artery. A key enzyme in thrombus formation is the serine protease thrombin, which is responsible for
Tomasz Pawlak et al.
The journal of physical chemistry. B, 116(6), 1974-1983 (2012-01-14)
DFT methods were employed to compute the (13)C NMR chemical shift tensor (CST) parameters for crystals of YAF peptides (Tyr-Ala-Phe) with different stereochemistry for the Ala residue. Tyr-D-Ala-Phe 1 crystallizes in the C2 space group while Tyr-L-Ala-Phe crystallizes in either
J Li et al.
Electrophoresis, 20(1), 171-179 (1999-03-05)
The separation of stereoisomers, particularly enantiomers, is important when their physiological activity differs. We have resolved the four stereoisomers each of alanylphenylalanine (Ala-Phe) and of leucylphenylalanine (Leu-Phe) by capillary electrophoresis using beta-cyclodextrin as a buffer additive and urea to enhance
Semra Kocabiyik et al.
Protein expression and purification, 73(2), 223-230 (2010-05-13)
In this study we describe, the construction of a co-expression vector allowing simultaneous production of Thermoplasma volcanium 20S proteasome alpha- and beta-subunits in Escherichia coli. This heterologous expression system provided high level production of fully active 20S proteasome that can
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.