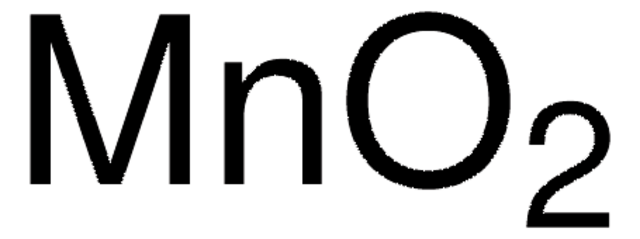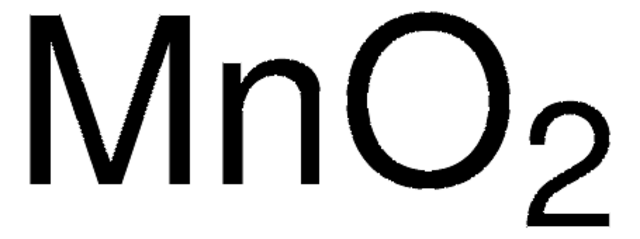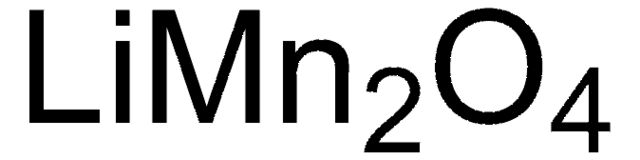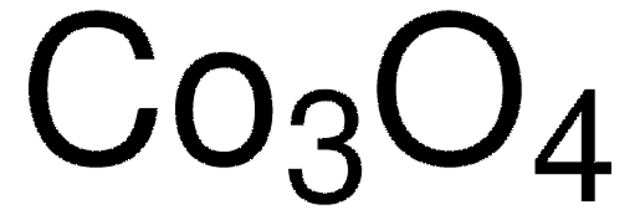310700
Manganese(IV) oxide
10 μm, reagent grade, ≥90%
Sinonimo/i:
Manganese dioxide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
MnO2
Numero CAS:
Peso molecolare:
86.94
Numero CE:
Numero MDL:
Codice UNSPSC:
12352303
ID PubChem:
NACRES:
NA.55
Saggio:
≥90%
Grado:
reagent grade
Stato:
powder
Prodotti consigliati
Grado
reagent grade
Livello qualitativo
Saggio
≥90%
Stato
powder
Dimensione particelle
10 μm
Punto di fusione
535 °C (dec.) (lit.)
Stringa SMILE
O=[Mn]=O
InChI
1S/Mn.2O
NUJOXMJBOLGQSY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Manganese(IV) oxide is an oxidizing reagent that can be used for the oxidation of propargylic alcohols, benzylic or heterocyclic alcohols, saturated alcohols, 1,2-diols, allylic alcohols to α, β-ethylenic aldehydes or ketones, and amines to aldehydes, imines, amides, and diazo compounds. It can also be used for the conversion of allylic alcohols to α, β-ethylenic esters or amides, hydration of nitriles to amides, dehydrogenation and aromatization reactions.
Applicazioni
- High-oxidation-state 3d metal complexes: Explores the catalytic properties of manganese(IV) oxide within high-oxidation-state complexes for advanced organic synthesis, demonstrating its critical role in accelerating chemical reactions and enhancing yield efficiencies, beneficial for pharmaceutical and chemical industries (Cheng J et al., 2018).
- Synthesis and properties of manganese complexes: Details the synthesis of new manganese complexes that demonstrate unique redox properties, useful for understanding electron transfer processes in various chemical and environmental contexts (Baffert C et al., 2002).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - STOT RE 2 Inhalation
Organi bersaglio
Brain
Codice della classe di stoccaggio
13 - Non Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 2
Punto d’infiammabilità (°F)
does not flash
Punto d’infiammabilità (°C)
does not flash
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Manganese dioxide
Cahiez G, et al.
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Wen-Hui Kuan et al.
Journal of hazardous materials, 239-240, 152-159 (2012-09-25)
This study examined the reaction of methylene blue (MB) with tunneled manganese oxide pyrolusite regarding pH and reaction time. MB was cleaved through N-demethylation, in which reaction azure B (AB), azure A (AA), azure C (AC), and thionin (TH) were
Heng Lai et al.
ACS applied materials & interfaces, 4(5), 2325-2328 (2012-05-02)
MnO(2) nanoflakes coated on carbon nanohorns (CNHs) has been synthesized via a facile solution method and evaluated as anode for lithium-ion batteries. By using CNHs as buffer carrier, MnO(2)/CNH composite displays an excellent capacity of 565 mA h/g measured at
Xihong Lu et al.
Advanced materials (Deerfield Beach, Fla.), 24(7), 938-944 (2012-03-10)
WO3–x@Au@MnO2 core–shell nanowires (NWs) are synthesized on a flexible carbon fabric and show outstanding electrochemical performance in supercapacitors such as high specific capacitance, good cyclic stability, high energy density, and high power density. These results suggest that the WO3–x@Au@MnO2 NWs
Y Wang et al.
Journal of colloid and interface science, 380(1), 8-15 (2012-06-02)
Bio-inspired chemical approach has been developed for the surface modification and electrophoretic deposition of manganese dioxide and zirconia nanoparticles, prepared by chemical precipitation methods. Caffeic acid, trans-cinnamic acid, p-coumaric acid, and 2,4-dihydroxycinnamic acid were investigated for the surface modification of
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.